Quinox, a Quinoline-Type N-Oxide, as Organocatalyst in the Asymmetric Allylation of Aromatic Aldehydes with Allyltrichlorosilanes: The Role of Arene–Arene Interactions†
Andrei V. Malkov Dr.
Department of Chemistry, Joseph Black Building, University of Glasgow, Glasgow G12 8QQ, UK, Fax: (+44) 141-33-488
Search for more papers by this authorLenka Dufková
Department of Chemistry, Joseph Black Building, University of Glasgow, Glasgow G12 8QQ, UK, Fax: (+44) 141-33-488
Department of Organic Chemistry, Charles University, 128 40 Prague 2, Czech Republic
Search for more papers by this authorLouis Farrugia Dr.
Department of Chemistry, Joseph Black Building, University of Glasgow, Glasgow G12 8QQ, UK, Fax: (+44) 141-33-488
Search for more papers by this authorPavel Kočovský Prof. Dr.
Department of Chemistry, Joseph Black Building, University of Glasgow, Glasgow G12 8QQ, UK, Fax: (+44) 141-33-488
Search for more papers by this authorAndrei V. Malkov Dr.
Department of Chemistry, Joseph Black Building, University of Glasgow, Glasgow G12 8QQ, UK, Fax: (+44) 141-33-488
Search for more papers by this authorLenka Dufková
Department of Chemistry, Joseph Black Building, University of Glasgow, Glasgow G12 8QQ, UK, Fax: (+44) 141-33-488
Department of Organic Chemistry, Charles University, 128 40 Prague 2, Czech Republic
Search for more papers by this authorLouis Farrugia Dr.
Department of Chemistry, Joseph Black Building, University of Glasgow, Glasgow G12 8QQ, UK, Fax: (+44) 141-33-488
Search for more papers by this authorPavel Kočovský Prof. Dr.
Department of Chemistry, Joseph Black Building, University of Glasgow, Glasgow G12 8QQ, UK, Fax: (+44) 141-33-488
Search for more papers by this authorWe thank the University of Glasgow, the Socrates Exchange program, and Dr. Alfred Bader for financial support.
Graphical Abstract
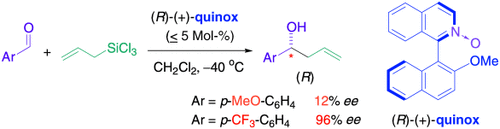
Die Allylierung aromatischer Aldehyde mit Allyltrichlorsilanen kann durch die neue Lewis-Base quinox organisch katalysiert werden. Bei elektronenarmen Aldehyden werden hohe Enantioselektivitäten beobachtet, bei elektronenreichen dagegen nur geringe (siehe Schema). Dies deutet darauf hin, dass die Aren-Aren-Wechselwirkung zwischen dem elektronenreichen Katalysator und dem angreifenden Aldehyd die enantiofaciale Selektivität bestimmt.
Supporting Information
Supporting information for this article is available on the WWW under http://www.wiley-vch.de/contents/jc_2001/2003/z51737_s.pdf or from the author.
Please note: The publisher is not responsible for the content or functionality of any supporting information supplied by the authors. Any queries (other than missing content) should be directed to the corresponding author for the article.
References
- 1
- 1aS. E. Denmark, D. M. Coe, N. E. Pratt, B. D. Griedel, J. Org. Chem. 1994, 59, 6161;
- 1bS. E. Denmark, X. Su, Y. Nishigaichi, J. Am. Chem. Soc. 1998, 120, 12 990;
- 1cS. E. Denmark, R. A. Stavenger, K.-T. Wong, X. Su, J. Am. Chem. Soc. 1999, 121, 4982;
- 1dS. E. Denmark, R. A. Stavenger, K.-T. Wong, X. Su, J. Am. Chem. Soc. 1999, 121, 4982;
- 1eS. E. Denmark, J. Fu, J. Am. Chem. Soc. 2000, 122, 12 021;
- 1fS. E. Denmark, S. M. Pham, Helv. Chim. Acta 2000, 83, 1846;
- 1gS. E. Denmark, J. Fu, J. Am. Chem. Soc. 2001, 123, 9488;
- 1hS. E. Denmark, Y. Fan, J. Am. Chem. Soc. 2002, 124, 4233;
- 1iS. E. Denmark, J. Fu, J. Am. Chem. Soc. 2003, 125, 2208. For overviews, see:
- 1jS. E. Denmark, R. A. Stavenger, Acc. Chem. Res. 2000, 33, 432;
- 1kS. E. Denmark, J. Fu, Chem. Commun. 2003, 167.
- 2aK. Iseki, Y. Kuroki, M. Takahashi, Y. Kobayashi, Tetrahedron Lett. 1996, 37, 5149;
- 2bK. Iseki, Y. Kuroki, M. Takahashi, S. Kishimoto, Y. Kobayashi, Tetrahedron 1997, 53, 3513.
- 3
- 3aM. Nakajima, M. Saito, M. Shiro, S. Hashimoto, J. Am. Chem. Soc. 1998, 120, 6419;
- 3bM. Nakajima, M. Saito, S. Hashimoto, Chem. Pharm. Bull. 2000, 48, 306;
- 3cM. Nakajima, M. Saito, S. Hashimoto, Tetrahedron: Asymmetry 2002, 13, 2449;
- 3dM. Nakajima, M. Saito, M. Uemura, S. Hashimoto, Tetrahedron Lett. 2002, 43, 8827.
- 4T. Shimada, A. Kina, S. Ikeda, T. Hayashi, Org. Lett. 2002, 4, 2799.
- 5A. V. Malkov, M. Orsini, D. Pernazza, K. W. Muir, V. Langer, P. Meghani, P. Kočovský, Org. Lett. 2002, 4, 1047.
- 6A. V. Malkov, M. Bell, M. Vassieu, V. Bugatti, P. Kočovský, J. Mol. Catal. A 2003, 196, 179.
- 7For a recent account on the face-to-face and center-to-edge interactions on aromatic systems and polar effects, see:
- 7aF. Cozzi, R. Annunziata, M. Benaglia, M. Cinquini, L. Raimondi, K. K. Baldridge, J. S. Siegel, Org. Biomol. Chem. 2003, 1, 157;
- 7bFor a recent review on the arene–arene, π stacking, and C-H/π interactions, see: E. A. Meyer, R. K. Castellano, F. Diederich, Angew. Chem. 2003, 115, 1244; Angew. Chem. Int. Ed. 2003, 42, 1210;
- 7cFor C-H/π interactions, see: P. Hobza, Z. Havlas, Chem. Rev. 2000, 100, 4253;
- 7dFor a review on π shielding in organic synthesis, see: G. B. Jones, Tetrahedron 2001, 57, 7999.
- 8The π stacking has also been proposed by Hayashi to account for the high catalytic activity of a chiral 2,2′-bipyridine-N,N-bisoxide.[4]
- 9
- 9aJ. M. Brown, D. I. Hulmes, T. P. Layzell, J. Chem. Soc. Chem. Commun. 1993, 1673;
- 9bN. W. Alcock, J. M. Brown, D. I. Hulmes, Tetrahedron: Asymmetry 1993, 4, 743.
- 10Š. Vyskočil, L. Meca, I. Tišlerová, I. Císařová, M. Polášek, S. R. Harutyunyan, Y. N. Belokon, R. M. J. Stead, L. Farrugia, S. C. Lockhart, W. L. Mitchell, P. Kočovský, Chem. Eur. J. 2002, 8, 4633.
10.1002/1521-3765(20021018)8:20<4633::AID-CHEM4633>3.0.CO;2-N CAS PubMed Web of Science® Google Scholar
- 11For the previous use of binol to resolve bipyridine-type N,N-bisoxides, see refs [3,4].
- 12While this work was in progress, Nakajima published the same synthesis of (+)-11, including the resolution with (S)-(−)-binol.[3d] However, this synthesis was mentioned as a footnote without specifying the conditions and the absolute configuration of (+)-11 was not determined.
- 13Crystal data for (R)-11⋅(S)-12: colorless crystals, space group P212121, a=8.5583(1) Å, b=11.9097(1) Å, c=29.0481(4) Å, V=2960.78(6) Å3, Z=4, dcalcd=1.318 g cm−3, μ=0.085 mm−1, RF(obs)=0.0395.
- 14Investigation of the allylation of 1 a with 2, catalyzed by (R)-(+)-11 of 29 %, 50 %, and 75 % ee, respectively, has demonstrated a fully linear relationship between the enantiopurity of the catalyst and the product. In these experiments, the resulting product 3 a was of 27 %, 45 %, and 71 % ee, respectively.
- 15Chloroform has been shown to be the solvent that most strongly stabilizes the arene–arene interactions: G. A. Breault, C. A. Hunter, P. C. Mayers, J. Am. Chem. Soc. 1998, 120, 3402.
- 16Y. Yamamoto, H. Yatagai, K. Maruyama, J. Am. Chem. Soc. 1981, 103, 1969.
- 17H. Doucet, M. Santelli, Tetrahedron: Asymmetry 2000, 11, 4163.
- 18U. S. Racherla, Y. Liao, H. C. Brown, J. Org. Chem. 1992, 57, 6614.
- 19J. Uenishi, T. Hiraoka, S. Hata, K. Nishiwaki, O. Yonemitsu, K. Nakamura, H. Tsukube, J. Org. Chem. 1998, 63, 2481.
- 20For a discussion on cyclic/open transition state, see:
- 20aS. E. Denmark, N. G. Almstead, J. Org. Chem. 1994, 59, 5130;
- 20bS. E. Denmark, S. Hosoi, J. Org. Chem. 1994, 59, 5133.
- 21Hayashi has proposed π–π stacking of 1 and the catalysts but, in his case, the variation of the enantioselectivity was less dramatic (94 % ee for 1 d and 56 % ee for 1 l as the extremes of the scale).[4] Furthermore, his catalyst gave the best results in MeCN, which is known not to support arene–arene interactions,[15] thus suggesting that his and our catalyst may operate through a different mode of interactions.
Citing Literature
This is the
German version
of Angewandte Chemie.
Note for articles published since 1962:
Do not cite this version alone.
Take me to the International Edition version with citable page numbers, DOI, and citation export.
We apologize for the inconvenience.