Dihedral-Angle Modulation of meso–meso-Linked ZnII Diporphyrin through Diamine Coordination and Its Application to Reversible Switching of Excitation Energy Transfer†
Hideyuki Shinmori Dr.
Department of Chemistry, Graduate School of Science, Kyoto University, and, Core Research for Evolutional Science and Technology (CREST), Japan Science and Technology Corporation, Sakyo-ku, Kyoto 606–8502, Japan, Fax: (+81) 75-753-3970
Search for more papers by this authorTae Kyu Ahn Dr.
Center for Ultrafast Optical Characteristics Control and, Department of Chemistry, Yonsei University, Seoul 120–749, Korea, Fax: (+82) 2-2123-2434
Search for more papers by this authorHyun Sun Cho Dr.
Center for Ultrafast Optical Characteristics Control and, Department of Chemistry, Yonsei University, Seoul 120–749, Korea, Fax: (+82) 2-2123-2434
Search for more papers by this authorDongho Kim Prof.
Center for Ultrafast Optical Characteristics Control and, Department of Chemistry, Yonsei University, Seoul 120–749, Korea, Fax: (+82) 2-2123-2434
Search for more papers by this authorNaoya Yoshida Dr.
Department of Chemistry, Graduate School of Science, Kyoto University, and, Core Research for Evolutional Science and Technology (CREST), Japan Science and Technology Corporation, Sakyo-ku, Kyoto 606–8502, Japan, Fax: (+81) 75-753-3970
Search for more papers by this authorAtsuhiro Osuka Prof.
Department of Chemistry, Graduate School of Science, Kyoto University, and, Core Research for Evolutional Science and Technology (CREST), Japan Science and Technology Corporation, Sakyo-ku, Kyoto 606–8502, Japan, Fax: (+81) 75-753-3970
Search for more papers by this authorHideyuki Shinmori Dr.
Department of Chemistry, Graduate School of Science, Kyoto University, and, Core Research for Evolutional Science and Technology (CREST), Japan Science and Technology Corporation, Sakyo-ku, Kyoto 606–8502, Japan, Fax: (+81) 75-753-3970
Search for more papers by this authorTae Kyu Ahn Dr.
Center for Ultrafast Optical Characteristics Control and, Department of Chemistry, Yonsei University, Seoul 120–749, Korea, Fax: (+82) 2-2123-2434
Search for more papers by this authorHyun Sun Cho Dr.
Center for Ultrafast Optical Characteristics Control and, Department of Chemistry, Yonsei University, Seoul 120–749, Korea, Fax: (+82) 2-2123-2434
Search for more papers by this authorDongho Kim Prof.
Center for Ultrafast Optical Characteristics Control and, Department of Chemistry, Yonsei University, Seoul 120–749, Korea, Fax: (+82) 2-2123-2434
Search for more papers by this authorNaoya Yoshida Dr.
Department of Chemistry, Graduate School of Science, Kyoto University, and, Core Research for Evolutional Science and Technology (CREST), Japan Science and Technology Corporation, Sakyo-ku, Kyoto 606–8502, Japan, Fax: (+81) 75-753-3970
Search for more papers by this authorAtsuhiro Osuka Prof.
Department of Chemistry, Graduate School of Science, Kyoto University, and, Core Research for Evolutional Science and Technology (CREST), Japan Science and Technology Corporation, Sakyo-ku, Kyoto 606–8502, Japan, Fax: (+81) 75-753-3970
Search for more papers by this authorThe work at Yonsei University was financially supported by the Creative Research Initiatives Program of the Ministry of Science and Technology of Korea. N.Y. thanks the JSPS Research Fellowship for Young Scientists.
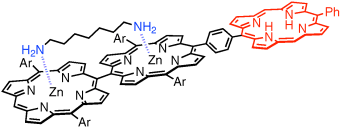
Graphical Abstract
Der Diederwinkel in meso-meso-verknüpften ZnII-Diporphyrinen wird durch verbrückende Koordination von 1,7-Diaminoheptan an beide Zinkzentren eingestellt (siehe Bild). Als Folge dieser mechanischen Einschränkung wird die Energie des S1-Zustands der Zinkdiporphyrin-Einheit abgesenkt, und die Richtung des Energietransfers im angeregten Zustand kehrt sich um; der Prozess kann durch Zugabe von Essigsäure rückgängig gemacht werden.
Supporting Information
Supporting information for this article is available on the WWW under http://www.wiley-vch.de/contents/jc_2001/2003/z51177_s.pdf or from the author.
Please note: The publisher is not responsible for the content or functionality of any supporting information supplied by the authors. Any queries (other than missing content) should be directed to the corresponding author for the article.
References
- 1
- 1aM. R. Wasielewski, Chem. Rev. 1992, 92, 435;
- 1bD. Gust, T. A. Moore, A. L. Moore, Acc. Chem. Res. 1993, 26, 198;
- 1cK. Maruyama, A. Osuka, Pure Appl. Chem. 1990, 62, 1511.
- 2
- 2aT. D. James, K. Sandanayake, S. Shinkai, Angew. Chem. 1994, 106, 2287; Angew. Chem. Int. Ed. Engl. 1994, 33, 2207;
- 2bR. Krämaer, Angew. Chem. 1998, 110, 804;
10.1002/(SICI)1521-3757(19980316)110:6<804::AID-ANGE804>3.0.CO;2-G Google ScholarAngew. Chem. Int. Ed. 1998, 37, 772;10.1002/(SICI)1521-3773(19980403)37:6<772::AID-ANIE772>3.0.CO;2-Z CAS PubMed Web of Science® Google Scholar
- 2cL. Fabbrizzi, M. Licchelli, P. Pallavicini, L. Parodi, Angew. Chem. 1998, 110, 838;
10.1002/(SICI)1521-3757(19980316)110:6<838::AID-ANGE838>3.0.CO;2-P Google ScholarAngew. Chem. Int. Ed. 1998, 37, 800.10.1002/(SICI)1521-3773(19980403)37:6<800::AID-ANIE800>3.0.CO;2-U CAS Web of Science® Google Scholar
- 3
- 3aD. Holten, D. F. Bocian, J. S. Lindsey, Acc. Chem. Res. 2002, 35, 57;
- 3bA. P. de Silva, N. D. McClenaghan, J. Am. Chem. Soc. 2000, 122, 3965;
- 3cA. P. de Silva, H. Q. N. Gunnaratne, C. P. McCoy, Nature 1993, 364, 42;
- 3dA. Credi, V. Balzani, S. Langford, J. F. Stoddart, J. Am. Chem. Soc. 1997, 119, 2679.
- 4
- 4aJ. Walz, K. Ulrich, H. Port, H. C. Wolf, J. Wonner, F. Effenberger, Chem. Phys. Lett. 1993, 213, 321;
- 4bH. Shiratori, T. Ohno, K. Nozaki, A. Osuka, Chem. Phys. Lett. 2000, 320, 631;
- 4cT. B. Norsten, N. R. Brabda, J. Am. Chem. Soc. 2001, 123, 1784;
- 4dA. Osuka, D. Fujikane, H. Shinmori, S. Kobatake, M. Irie, J. Org. Chem. 2001, 66, 3913;
- 4eJ. L. Bahr, G. Kodis, L. de la Garza, S. Lin, A. L. Moore, T. A. Moore, D. Gust, J. Am. Chem. Soc. 2001, 123, 7124;
- 4fP. A. Liddell, G. Kodis, A. L. Moore, T. A. Moore, D. Gust, J. Am. Chem. Soc. 2002, 124, 7668.
- 5
- 5aA. Osuka, H. Shimidzu, Angew. Chem. 1997, 109, 93;
10.1002/ange.19971090126 Google ScholarAngew. Chem. Int. Ed. Engl. 1997, 36, 135;
- 5bA. Nakano, A. Osuka, I. Yamazaki, T. Yamazaki, Y. Nishimura, Angew. Chem. 1998, 110, 3172;
10.1002/(SICI)1521-3757(19981102)110:21<3172::AID-ANGE3172>3.0.CO;2-5 Web of Science® Google ScholarAngew. Chem. Int. Ed. 1998, 37, 3023;10.1002/(SICI)1521-3773(19981116)37:21<3023::AID-ANIE3023>3.0.CO;2-A CAS PubMed Web of Science® Google Scholar
- 5cN. Aratani, A. Osuka, Y. H. Kim, D. H. Jeong, D. Kim, Angew. Chem. 2000, 112, 1517;
10.1002/(SICI)1521-3757(20000417)112:8<1517::AID-ANGE1517>3.0.CO;2-D Google ScholarAngew. Chem. Int. Ed. 2000, 39, 1458;10.1002/(SICI)1521-3773(20000417)39:8<1458::AID-ANIE1458>3.0.CO;2-E CAS PubMed Web of Science® Google Scholar
- 5dY. H. Kim, D. H. Jeong, D. Kim, S. C. Jeoung, H. S. Cho, S. Kim, N. Aratani, A. Osuka, J. Am. Chem. Soc. 2001, 123, 76.
- 6
- 6aN. Yoshida, A. Osuka, Org. Lett. 2000, 2, 2963;
- 6bN. Yoshida, T. Ishizuka, A. Osuka, D. H. Jeong, H. S. Cho, D. Kim, Y. Matsuzaki, A. Nogami, K. Tanaka, Chem. Eur. J. 2003, 9, 58.
- 7J. K. M. Sanders in The Porphyrin Handbook, Vol. 3 (Ed ), Academic Press, San Diego, 2000, pp. 347–368.
- 7a Examples of conformational changes induced by the coordination of diamines to ZnII diporphyrins; C. A. Hunter, M. N. Meah, J. K. M. Sanders, J. Chem. Soc. Chem. Commun. 1988, 692;
- 7bH. L. Anderson, C. A. Hunter, J. K. M. Sanders, J. Chem. Soc. Chem. Commun. 1989, 226;
- 7cI. P. Danks, T. G. Lane, I. O. Sutherland, M. Yap, Tetrahedron 1992, 48, 7679;
- 7dM. J. Crossley, L. G. Mackay, A. C. Try, J. Chem. Soc. Chem. Commun. 1995, 1925;
- 7eX. Huang, B. H. Rickman, B. Borhan, N. Berova, K. Nakanishi, J. Am. Chem. Soc. 1998, 120, 6185.
- 8
- 8aX. Zhou, K. S. Chan, J. Org. Chem. 1998, 63, 99;
- 8bA. G. Hyslop, M. A. Kellett, P. M. Ivovine, M. J. Therien, J. Am. Chem. Soc. 1998, 120, 12 676;
- 8cL. Yu, J. S. Lindsey, Tetrahedron 2001, 57, 9285;
- 8dN. Aratani, A. Osuka, Org. Lett. 2001, 3, 4213.
- 9The observed fluorescence upon addition of acetic acid was the same as that of nonprotonated free-base porphyrin, thus excluding the formation of dicationic species in toluene solution. As about 1000-fold excess of acetic acid was needed to recover the fluorescence of the free base porphyrin in 7 DA–4, therefore the switching cycle only occurs once at the present stage.
Citing Literature
This is the
German version
of Angewandte Chemie.
Note for articles published since 1962:
Do not cite this version alone.
Take me to the International Edition version with citable page numbers, DOI, and citation export.
We apologize for the inconvenience.