A Comparison Between Early Presentation of Dementia with Lewy Bodies, Alzheimer's Disease, and Parkinson's Disease: Evidence from Routine Primary Care and UK Biobank Data
Anette Schrag, Stanley Durrleman, and Jean-Christophe Corvol contributed equally to this study.
Abstract
Objective
The purpose of this study was to simultaneously contrast prediagnostic clinical characteristics of individuals with a final diagnosis of dementia with Lewy Bodies (DLB), Parkinson's disease (PD), and Alzheimer's disease (AD) compared with controls without neurodegenerative disorders.
Methods
Using the longitudinal THIN database in the United Kingdom, we tested the association of each neurodegenerative disorder with a selected list of symptoms and broad families of treatments, and compared the associations between disorders to detect disease-specific effects. We replicated the main findings in the UK Biobank.
Results
We used data of 28,222 patients with PD, 20,214 with AD, 4,682 with DLB, and 20,214 healthy controls. All neurodegenerative disorders were significantly associated with the presence of multiple clinical characteristics before their diagnosis, including sleep disorders, falls, psychiatric symptoms, and autonomic dysfunctions. When comparing patients with DLB with patients with PD and patients with AD patients, falls, psychiatric symptoms, and autonomic dysfunction were all more strongly associated with DLB in the 5 years preceding the first neurodegenerative diagnosis. The use of statins was lower in patients who developed PD and higher in patients who developed DLB compared to patients with AD. In patients with PD, the use of statins was associated with the development of dementia in the 5 years following PD diagnosis.
Interpretation
Prediagnostic presentations of falls, psychiatric symptoms, and autonomic dysfunctions were more strongly associated with DLB than PD and AD. This study also suggests that although several associations with medications are similar in neurodegenerative disorders, statin usage is negatively associated with PD but positively with DLB and AD as well as development of dementia in PD. ANN NEUROL 2023;94:259–270
Graphical Abstract
Neurodegenerative diseases represent one of the main public health issues in Western societies. In 2019, about 57 million individuals were living with dementia worldwide.1 Alzheimer's disease (AD) accounts for 60% to 70% of dementia cases (29 million) and represents the first cause of dementia, whereas dementia related to synucleinopathies (Parkinson's disease [PD] dementia and dementia with Lewy bodies [DLB]) are the second most common neurodegenerative causes of dementia.2 PD is the second most common cause of neurodegenerative disease, estimated worldwide to affect 6.1 million individuals in 2016.3 All these neurodegenerative diseases are known to have a very long preclinical stage. For AD, accumulation of amyloid can precede a clinical diagnosis of dementia by up to 20 years.4 Synucleinopathies, including PD and DLB, also have a long prodromal stage, as demonstrated by isolated rapid eye movement (REM) sleep behavioral disorders, which can precede symptomatic PD or DLB by 10 years or more.5 The first dysautonomic signs have been estimated to start as early as 15 years before motor symptoms in PD.6, 7
Neurological disorders are often underdiagnosed in the primary care setting.8 Analyses on general practitioners’ (GPs) databases have already led to the identification of disease-specific prodromal symptoms of PD and AD as well as risk factors in the primary care data.9, 10 However, it is unknown how the prodromal features and risk factors of these disorders differ. Analyses on the prodrome of DLB have so far been limited to core symptoms of DLB.11, 12 Overall, cross-disorder analyses focusing on shared and specific associations with neurodegenerative diseases are rare.13 We undertook cross-disorder analyses in order to identify prediagnostic associations and risk factors of the most common neurodegenerative conditions (AD, PD, and DLB), using data collected by GPs over a long-time window. We sought to identify disorders and drug prescriptions associated with AD, PD, and DLB, as well as those that are specific to each condition. Predictors of dementia in PD observable in primary care are also missing.14 In a secondary analysis, we aimed at understanding which drug prescriptions are associated with the presence of dementia in the 5 years following a first PD diagnosis. We relied on the Health Improvement Network UK primary care for discovery and the UK Biobank (UKB) for selected replication.
Methods
Study Design and Participants
THIN Database
We used The Health Improvement Network (THIN) database,15 a large European standardized database of anonymized electronic medical records collected at the physicians' level by the company Cegedim and coded using the International Classification of Diseases, 10th Revision (ICD-10) codes. Data were collected in 400 general practices, representing around 6% of the UK population.9 Several reports have shown that electronically coded diagnoses in this database are representative of the UK general practice population in terms of demographics and type of consultation.15, 16 For each patient and visit, we obtained the diagnosis and prescription established during the visit, as well as all ongoing prescriptions and associated diagnoses.
We defined our neurological cases based on ICD-10 codes given by the GPs of the THIN database recorded between January 1996 and April 2020. For the neurodegenerative disorders of interest, AD cases corresponded to codes F00 and G30, PD cases to code G20, and DLB cases to code G31.8 (and READ code Eu025, to exclude frontotemporal dementia). We extracted all patients with DLB in the database, and a random subsample of patients with AD or with PD, with at least 2 years of follow-up. We defined age at onset as the age at the first record coded with AD, PD, or DLB diagnosis. Controls were screened for any history of AD (F00 et G30), PD (G20), frontotemporal dementia (G31.0 and G31.8), DLB (G31.8), Huntington's disease (G10), or multiple sclerosis (G35). We had access to 20,214 controls matched with AD cases on sex and age +/− 1 year at last record in the database from a precedent study which was specific to AD.10
UK Biobank Replication Sample
For replication, we used the UKB, a large population-based cohort which consists of 502,492 unselected volunteers from the United Kingdom, recruited between 2006 and 2010, of which 487,409 underwent genotyping and extensive phenotyping.17 Phenotypic data of interest include age, sex, body mass index, Townsend deprivation index, and the age they completed full time education. Cholesterol data was measured from blood collected in the baseline visit. Informed consent was obtained from all participants registered in the UKB.
In the UKB, we included the 1,005 patients with a reported diagnosis of AD, the 2,268 patients with PD, and the 2,732 patients with a report of dementia. The clinical outcomes (Parkinson's field ID 42030, Alzheimer's field ID 42020, all dementia field ID 42018) were generated by the UKB (category 42), by crossing UKB baseline self-reports with linked hospital admissions and death register data. We excluded from further analysis the 69 patients who had records of both AD and PD diagnoses (see Tables S7 and S8 for statistics on this population). Finally, we only considered patients with at least one UKB visit before a diagnosis of neurodegenerative disease.
Ethics Committee Approval
Approval was sorted before carrying out this study (and for the use of datasets). The study was a retrospective analysis of secondary anonymized patient data only. For the UK THIN database, data are only available to researchers carrying out approved medical research. Ethical approval was granted by the National Health System (NHS) South-East Multicentre Research Ethics Committee in 2003 (ref: 03/01/073) for establishment of the THIN database, and it was updated in 2011. A further update and approval were granted in 2020 by the NHS South Central, Oxford C Research Ethics Committee (Ref: 20/SC/0011). The study was approved by the THIN Scientific Research Committee (SRC; SRC reference 20-002). Researchers willing to replicate the results should address a request to the Cegedim company ([email protected]). Informed consent was obtained from all UKB participants. Procedures are controlled by a dedicated Ethics and Guidance Council (http://www.UKBiobank.ac.uk/ethics), with the Ethics and Governance Framework available at http://www.UKBiobank.ac.uk/wp-content/uploads/2011/05/EGF20082.pdf. Institutional review board (IRB) approval was also obtained from the North West Multi-Centre Research Ethics Committee. This research has been conducted using the UK Biobank Resource under Application Number 53185.
Health Conditions and Prescriptions of Drugs of Interest
We considered symptoms previously reported to be associated with one of these neurodegenerative diseases in the literature and likely to be coded by a GP. We included in the analysis memory problems, tremor, confusion, hallucinations, sleep disorders, constipation, anxiety, depression, falls, hypotension, urinary tract disorders, and abnormal weight loss. We used the READ codes and the ICD-10 to extract health conditions (the mapping is provided in Table S16). We also conducted analysis on a preselected list of classes of medications previously reported to be associated with one of the neurodegenerative diseases, including all classes of antihypertensive medications, laxatives, statins, and medications used for neurological disorders, including treatments against depression, anxiety, and psychosis (Table S17). We relied on the Anatomical Therapeutic Chemical (ATC) Classification System18, 19 available directly in the THIN database. In the UKB, we used the medication-use data collected by trained nurses during interviews. The UKB classifies medications into 6,745 categories of which 1,809 were reported by 10 or more people.18 We mapped the medication categories into ATC codes (1,752 over 1,809 were mapped) as previously done in a genetic analysis of the UKB.18
Statistical Analysis
We first used the THIN database to estimate the association between the preselected list of health conditions or medication classes and each specific neurodegenerative disorder. We estimated the odds ratios (ORs) using a logistic regression corrected for sex and age at index date. We reported the OR estimates for different time windows prior to the neurodegenerative onset: 0 to 5 years, 5 to 10 years, and 10 to 15 years, in order to show their progression over time. The index date for each AD-control pair was defined as the first date of AD diagnosis of the corresponding patient with AD. The index date for PD and DLB was defined as age of onset. We corrected for multiple comparison using Bonferroni corrections. This led to using a significance threshold of p < 0.004 (p = 0.05 with Bonferroni correction for 12 potential exposures, 2-tailed test). All analyses were conducted using the StatsModel library in Python. Next, we compared the ORs among the 3 neurodegenerative disorders (PD vs. AD, DLB vs. AD, and DLB vs. PD). We also compared the association of prediagnostic medication use between patients with PD who developed dementia in the 5 years after a first PD diagnosis and those who did not. For this analysis, we excluded patients with a diagnosis of dementia before a first PD diagnosis. We compared these 2 groups in terms of medication usage in the 0 to 5 years before PD diagnosis.
In the UKB, we compared the AD and PD groups to replicate the significant results for all classes of medications considered in the THIN study. We used logistic models and controlled for sex, assessment center, age at diagnosis, age at entry in the cohort, and also for the Townsend deprivation index, body mass index, and education level. In a sensitivity analysis, we contrasted patients with PD with the group of patients with a report of AD or all field dementia. When testing associations of PD, AD, or all field dementia with the class of lipid-modifying agents, we further corrected for Apolipoprotein E (APOE) status (carriers of at least one epsilon 4 variant). We finally compared the associations with cholesterol, the LDL and HDL levels between patients with PD and patients with AD.
Results
In the THIN database, our full initial analytical cohort included 20,214 patients with a final diagnosis of AD, 28,222 patients with a diagnosis of PD, 4,682 patients with a diagnosis of DLB, and 20,214 healthy control patients. Table 1 describes the characteristics of the different populations. The median age at diagnosis was younger for PD (77 [70–83] years) than for DLB (80 [74–85] years) and patients with AD (82 [76–87] years) and for healthy controls (82 [76–87] years). There were more men than women in the PD and DLB cohorts (sex ratio of 57%) and the opposite in the AD and controls cohorts (sex ratio of 31%). There were 13,625 patients with AD, 14,539 patients with PD, 2,279 patients with DLB, and 10,205 healthy controls who had data available for more than 10 years before diagnosis. Demographic characteristics were similar between the different retrospective follow-up intervals (0–5, 10 or 15 years before diagnosis, Table S1). Only 5% of patients with AD had one prescription of antiparkinsonian drugs, whereas 76% of patients with PD had at least 2 prescriptions of antiparkinsonian drugs (Table S2).
| Characteristics | Total | With ≥5 years of retrospective data | ||||||
|---|---|---|---|---|---|---|---|---|
| Alzheimer's disease (n = 20,214) | Parkinson's disease (n = 28,222) | Dementia with Lewy Bodies (n = 4,682) | Healthy controls (n = 20,214) | Alzheimer's disease (n = 17,871) | Parkinson's disease (n = 22,281) | Dementia with Lewy Bodies (n = 2,712) | Healthy controls (n = 15,361) | |
| Sex: Women | 14,106 (69%) | 12,332 (43%) | 2050 (43%) | 14,106 (69%) | 12,531 (70%) | 9,532 (42%) | 1,156 (42%) | 10,624 (69%) |
| Men | 6,108 (31%) | 15,890 (57%) | 2,632 (57%) | 6,108 (31%) | 5,340 (30%) | 12,749 (58%) | 1,556 (58%) | 4,737 (31%) |
| Birth year | 1926 (1921–1933) | 1929 (1921–1938) | 1932 (1926–1938) | 1925 (1918–1933) | 1927 (1921–1933) | 1931 (1923–1940) | 1932 (1927–1939) | 1927 (1920–1934) |
| Age at index date | 82 (76–87) | 77 (70–83) | 80 (74–85) | 82 (76–87) | 82 (76–87) | 77 (70–83) | 79 (74–84) | 82 (76–87) |
| Person-years of data available before index date | 13 (8–18) | 10 (6–16) | 10 (1–18) | 10 (5–17) | 14 (10–18) | 12 (9–18) | 17 (12–21) | 13 (9–18) |
| Number of visits per year | 7 (4–11) | 7 (4–12) | 10 (6–17) | 7 (3–11) | 7 (4–10) | 7 (4–11) | 7 (4–11) | 7 (4–11) |
- Note: The data shown are numbers (%) and medians (IQR).
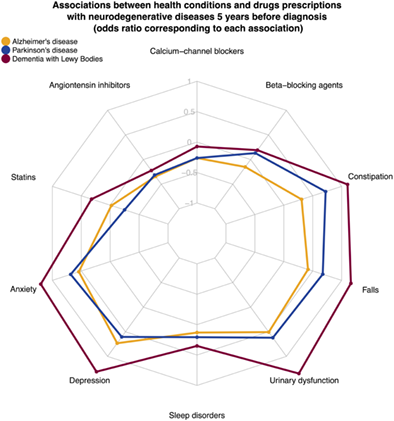
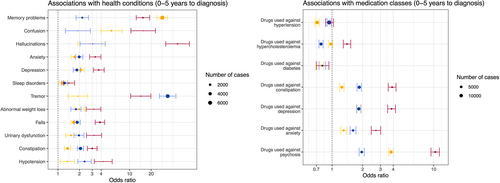
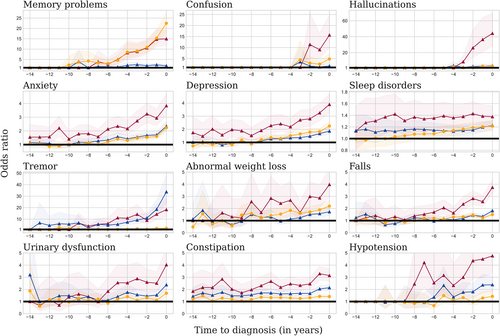
All conditions were more frequent than in controls in all 3 neurodegenerative diseases during the period 0 to 5 years before diagnosis (Fig 1). The prevalence of tremor, sleep disorders, constipation, anxiety, and urinary tract disorders was higher in patients with PD and patients with DLB than in controls up to 15 years prior diagnosis, and anxiety and depression only for patients with DLB (Table S3). The pre-diagnosis profile of the 12 health conditions investigated in the THIN study in AD, PD, DLB, and controls over time are shown in Figure 2 (Table S4). Treatments against depression, anxiety, and constipation were all more frequently prescribed in individuals with a later diagnosis of AD, PD, and DLB (see Fig 1). A higher frequency of benzodiazepines and serotonin reuptake inhibitors prescription was observed in the years preceding the diagnosis of all 3 diseases (Table 2). Lower antidiabetic medications use, including insulins, biguanides, sulfonylureas, and DDP4 inhibitors, was observed before the diagnosis of AD and PD (see Table 2). A lower prescription of agents acting on the renin–angiotensin–aldosterone system and selective beta-2-adrenoreceptor agonists was significantly observed before the diagnosis of the 3 diseases when compared to the healthy controls (see Table 2).
| Medications | THIN analysis | UK Biobank replication sample | ||||
|---|---|---|---|---|---|---|
| Alzheimer's disease | Parkinson's disease | Lewy Body disease | Parkinson's disease against Alzheimer's disease | Parkinson's disease against Alzheimer's disease | Parkinson against all dementia | |
| Diuretics | 0.74 (0.71, 0.77) | 0.93 (0.89, 0.97) | 0.85 (0.78, 0.93) | 1.25 (1.2, 1.31) | 0.88 (0.67, 1.14) | 0.85 (0.68, 1.07) |
| Beta-blocking agents | 0.86 (0.82, 0.9) | 1.14 (1.08, 1.19) | 1.21 (1.11, 1.33) | 1.32 (1.26, 1.38) | 0.88 (0.68, 1.13) | 0.78 (0.62, 0.97) |
| Calcium-channel blockers | 0.77 (0.73, 0.81) | 0.77 (0.73, 0.81) | 0.93 (0.85, 1.02) | 1.01 (0.96, 1.07) | 0.84 (0.64, 1.09) | 0.69 (0.55, 0.87) |
| Agent acting on angiotensin system | 0.71 (0.68, 0.74) | 0.73 (0.7, 0.77) | 0.8 (0.73, 0.87) | 1.05 (1.0, 1.1) | 0.93 (0.76,1.16) | 0.74 (0.62, 0.89) |
| Statins | 0.98 (0.93, 1.02) | 0.78 (0.75, 0.82) | 1.38 (1.27, 1.51) | 0.82 (0.78, 0.86) | 0.77 (0.63, 0.94) | 0.73 (0.62, 0.86) |
| Fibrates | 0.83 (0.65, 1.07) | 0.81 (0.64, 1.04) | 0.64 (0.37, 1.1) | 0.97 (0.76, 1.23) | 0.47 (0.16, 1.33) | 0.54 (0.21, 1.36) |
| Other lipid modifying agents | 0.83 (0.7, 0.99) | 0.82 (0.69, 0.98) | 1.05 (0.75, 1.45) | 0.95 (0.8, 1.14) | 1.08 (0.75, 1.56) | 1.02 (0.75, 1.38) |
| Insulins and analogs | 0.72 (0.61, 0.85) | 0.74 (0.63, 0.87) | 1.04 (0.78, 1.37) | 1.02 (0.86, 1.21) | 0.59 (0.35, 1.0) | 0.49 (0.31, 0.77) |
| Biguanides | 0.77 (0.71, 0.84) | 0.77 (0.7, 0.83) | 0.89 (0.76, 1.05) | 0.99 (0.91, 1.08) | 0.78 (0.53, 1.15) | 0.61 (0.44, 0.85) |
| Sulfonylureas | 0.76 (0.69, 0.84) | 0.83 (0.75, 0.91) | 0.64 (0.52, 0.79) | 1.06 (0.96, 1.17) | 1.21 (0.67, 2.17) | 0.75 (0.47, 1.2) |
| DDP4 inhibitors | 0.62 (0.48, 0.79) | 0.54 (0.42, 0.68) | 0.86 (0.57, 1.3) | 0.93 (0.72, 1.21) | ** | ** |
| GLP-1 inhibitors | 0.69 (0.36, 1.34) | 0.67 (0.36, 1.22) | 1.36 (0.53, 3.5) | 1.01 (0.53, 1.94) | ** | ** |
| Laxatives | 1.25 (1.19, 1.31) | 1.85 (1.76, 1.95) | 3.9 (3.57, 4.26) | 1.46 (1.39, 1.53) | 1.31 (0.85, 2.03) | 1.08 (0.75, 1.55) |
| Serotonin inhibitors | 1.84 (1.76, 1.93) | 1.83 (1.74, 1.92) | 3.85 (3.52, 4.2) | 0.95 (0.91, 0.99) | 0.81 (0.63, 1.04) | 0.88 (0.71, 1.09) |
| Dopamine antagonists | 2.42 (1.95, 3.0) | 2.18 (1.73, 2.74) | 4.25 (3.12, 5.79) | 0.79 (0.66, 0.94) | ** | ** |
| Benzodiazepines | 1.3 (1.22, 1.39) | 1.61 (1.5, 1.72) | 2.7 (2.43, 3.01) | 1.22 (1.15, 1.29) | 1.08 (0.49, 2.35) | 0.95 (0.51, 1.77) |
| Selective beta-2-adrenoreceptor agonists | 0.74 (0.7, 0.78) | 0.71 (0.67, 0.75) | 0.83 (0.74, 0.93) | 0.95 (0.89, 1.01) | 0.83 (0.6, 1.15) | 0.82 (0.62, 1.09) |
- Note: The p values are corrected for multiple comparisons in the THIN analysis. In the THIN analysis, odds ratios are corrected for age at diagnosis and sex and in the UK Biobank replication sample, for age at diagnosis, age at first visit, sex, center, BMI, education level and Townsend deprivation index. **Cannot be calculated because an insufficient number of presentations was recorded.
Cross Disorder Analyses
The odds of the 12 health conditions before diagnosis were different when comparing between diseases (see Table S4). In the 5 years prior to diagnosis, memory problems were more frequent in patients with AD compared to patients with PD and patients with DLB, whereas constipation was more common before PD and DLB onset than before AD onset. When comparing PD and DLB, we found that tremor was more frequent in the years preceding PD diagnosis, whereas memory troubles, confusion, hallucinations, constipation, falls, and depression were more frequent before the diagnosis of DLB. Laxatives were used more frequently prior to diagnosis of PD and DLB than of AD, in line with the higher prevalence of constipation in these conditions (see Table 2, Table S3). A higher beta-blocker agent prescription rate was observed in the years prior to PD and DLB diagnoses compared with patients with AD. A higher prescription rate of statins was observed in the years prior to the DLB diagnosis, whereas the opposite was observed prior to PD diagnosis, when compared with AD (see Table 2). Similar results were obtained when restricting the PD population to patients with at least one prescription of antiparkinsonian drug (see Fig S4).
Patients with Dementia with Lewy Bodies
Patients with DLB had stronger associations with most health conditions than patients with AD and patients with PD. DLB was strongly associated with depression, anxiety, autonomic dysfunction, constipation, urinary disorders, and hypotension. The association with falls was also stronger in the DLB cohort than in the other neurodegenerative disorders (see Fig 1). Neuroleptics were more frequently prescribed before DLB than AD and PD diagnoses, in accordance with their greater association with hallucination and confusion.
Associations with the Risk of Developing Dementia after a Parkinson's Disease Diagnosis
To investigate whether the observed associations we describe above were specific to the disease or to the syndrome (dementia), we ran a subsequent analysis in the subpopulation of patients with PD, comparing patients with PD with a subsequent diagnosis of dementia and those who remained free of dementia 5 years after PD diagnosis. The median age at diagnosis of PD was younger for patients with PD who did not develop dementia in the 5 years after the first PD diagnosis (73 [65–79] years) than for patients with PD who developed a dementia (79 [73–83] years; see Table S7, p < 0.0001). There were more men than women in both groups (56% in the PD group without dementia and 59% in the PD group with dementia). When comparing the 2 groups, after correcting for sex and age at diagnosis, the prescriptions of drugs against hypercholesterolemia, constipation, depression, and psychosis were individually associated with the development of a dementia in the 5 years after PD diagnosis (Fig 3). They all remained significantly associated with dementia in the multivariable model (Table 3). By contrast to what was found in the whole population, drugs against hypertension and anxiety were not individually associated with dementia after a PD diagnosis.
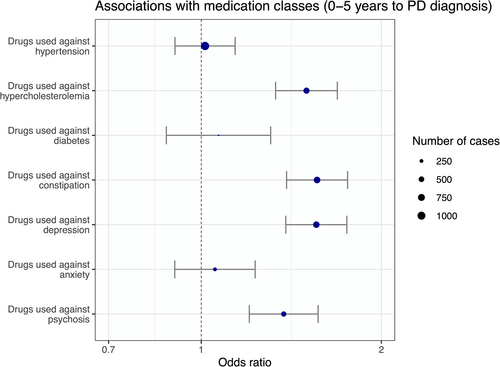
| Prevalence of at least one prescription in the 5 years before PD diagnosis | Univariable analysis | Multivariable analysis | ||||
|---|---|---|---|---|---|---|
| PD patients with dementia in the 5 years after PD diagnosis (n = 1,682) | PD patients without dementia in the 5 years after PD diagnosis (n = 6,924) | OR (95% CI) | p | OR (95% CI) | p | |
| Drugs used against hypertension | 1,074 (63.85%) | 4,126 (59.59%) | 1.01 (0.9, 1.14) | 0.805 (5.633) | 0.85 (0.75, 0.96) | 0.01 |
| Drugs used against hypercholesterolemia | 579 (34.42%) | 1814 (26.2%) | 1.5 (1.33, 1.69) | <0.001 (<0.001) | 1.49 (1.31, 1.69) | <0.001 |
| Drugs used against diabetes | 140 (8.32%) | 535 (7.73%) | 1.07 (0.87, 1.31) | 0.517 (3.62) | 0.9 (0.73, 1.11) | 0.334 |
| Drugs used against constipation | 671 (39.89%) | 1,685 (24.34%) | 1.56 (1.39, 1.76) | <0.001 (<0.001) | 1.42 (1.25, 1.6) | <0.001 |
| Drugs used against depression | 653 (38.82%) | 2,212 (31.95%) | 1.56 (1.38, 1.75) | <0.001 (<0.001) | 1.44 (1.27, 1.63) | <0.001 |
| Drugs used against anxiety | 255 (15.16%) | 1,112 (16.06%) | 1.05 (0.9, 1.23) | 0.504 (3.53) | 0.85 (0.72, 1.0) | 0.044 |
| Drugs used against psychosis | 430 (25.56%) | 1,262 (18.23%) | 1.37 (1.2, 1.57) | <0.001 (<0.001) | 1.23 (1.08, 1.42) | 0.003 |
- Note: In the multivariable model, we included all 7 health conditions. The treatment has to be prescribed in 0 to 5 years before Parkinson's disease diagnosis. Analyses are corrected for age at PD diagnosis and sex. The p values are corrected for multiple comparisons in the univariable analysis.
- Abbreviations: CI = confidence interval; OR = odds ratio; PD = Parkinson's disease.
UKB Replications
We used the UKB database to replicate the comparison in terms of medication usage between patients with AD and patients with PD (Table 4). A lower prescription of statins was also observed in the years prior to diagnosis of PD as compared to AD (OR = 0.77, p = 0.01). As in the THIN database, there was no significant difference between patients with AD and patients with PD regarding agents acting on the renin–angiotensin–aldosterone system. When patients with PD were compared with dementia cases (including vascular dementia and AD), prescriptions of all families of antihypertensives except diuretics (calcium channel blockers, beta blockers, and agents acting on renin–angiotensin–aldosterone system) and statins (see Table 2) were higher in individuals with subsequent dementia. We found that a higher prescription of statins was significantly associated with increased body mass index, lower age at diagnosis, and lower age at first visit in both the AD and PD cohorts but not significantly associated with any APOE genotypes (Table S13). The levels of LDL and HDL did not differ between patients with PD and patients with AD (Table S14), but we do not know if patients were on cholesterol-lowering medications and the number of patients who had a blood sample before their diagnosis was very small (88 patients with PD and 91 patients with AD).
| Characteristics | All | With at least one visit before diagnosis | ||||
|---|---|---|---|---|---|---|
| Total UK Biobank | All dementias | Alzheimer's disease | Parkinson's disease | Alzheimer's disease | Parkinson's disease | |
| Total | 502,492 | 2,732 | 1,005 | 2,268 | 920 | 1,272 |
| Women (n, %) | 273,377 (54%) | 1,216 (44%) | 491 (48%) | 879 (38%) | 465 (50%) | 505 (39%) |
| Men (n, %) | 229,115 (46%) | 1,516 (56%) | 514 (52%) | 1,389 (62%) | 455 (50%) | 767 (61%) |
| BMI | 26.86 (24.23–29.98) | 27.24 (24.41–30.3) | 26.75 (23.96–29.54) | 27.25 (24.7–30.16) | 26.66 (23.79–29.37) | 27.38 (24.75–30.26) |
| Education level | 16 (15–17) | 15 (15–16) | 15 (15–16) | 16 (15–17) | 15 (15–16) | 16 (15–17) |
| Townsend deprivation | −2.14 (−3.64–0.55) | −1.67 (−3.46–1.71) | −1.99 (−3.62–1.31) | −2.29 (−3.71–0.7) | −2.02 (−3.64–1.24) | −2.17 (−3.67–0.88) |
| Year of birth | 1950 (1945–1958) | 1943 (1940–1946) | 1942 (1940–1946) | 1944 (1941–1948) | 1942 (1940–1946) | 1944 (1941–1948) |
| Age at first visit | 58 (51–64) | 66 (62–68) | 66 (63–68) | 64 (60–67) | 66 (63–68) | 65 (61–68) |
| Age at diagnosis | 71 (67–74) | 72 (68–74) | 65 (58–71) | 72 (69–74) | 70 (66–73) | |
- Note: The data shown are numbers (%) or medians (IQR).
- Abbreviations: BMI = body mass index; IQR = interquartile range.
Discussion
In this large cross-disorder analysis, we compared the prodromal symptoms and drug prescriptions before the diagnosis of three of the main neurodegenerative diseases (AD, PD, and DLB). Our results complement previous approaches which tested separately a range of prodromal features on PD,9 AD,20 or DLB,11 observable at the GP level. By contrasting AD with PD and DLB, we found cardinal motor or non-motor symptoms of each disease to be more frequent than in controls at least 5 years before diagnosis: memory problems for patients with AD, tremor for patients with PD, and hallucinations, confusion, movement disorders, and memory problems for patients with DLB. We also observed that GPs reported more falls in future patients with DLB than in patients with AD and patients with PD in keeping with the McKeith 2017 criteria for DLB.21 We also found a robust association among all 3 neurological disorders and constipation in accordance with previous studies,22 even though the association was stronger for PD and DLB than for AD. More generally, we showed that all neurological disorders present more autonomic dysfunction in primary care compared to healthy controls, including constipation, urinary disorders, and hypotension several years before diagnosis, with a stronger association for PD and DLB than for AD. These autonomic dysfunctions might be used by primary care physicians in the future to anticipate a neurodegenerative disease. This result may support the hypothesis that mechanisms of neurodegenerative diseases pathological affect the periphery before spreading into the brain.23 These autonomic dysfunctions might alert the GP to a potential evolution toward a neurodegenerative disease.
We also investigated drug use and found a lower prescription of all the main antihypertensive families, including agents acting on the renin–angiotensin–aldosterone system (for all 3 diseases), calcium channel blockers (for AD and PD), and diuretics (for AD and DLB), in accordance with previous epidemiological studies showing an inverse association between antihypertensive drugs and AD, PD, or DLB.24 These results may support the hypothesis that vascular diseases are a risk factor of neurodegenerative diseases,24 a frequent comorbid condition of these diseases, and that decreasing cardiovascular risks by prescribing antihypertensive drugs would help to decrease the risk of developing all neurodegenerative diseases. Alternatively, some of these drugs may have unspecific neuroprotective effects,25 as antidiabetics drugs that were less prescribed in the years preceding all 3 diseases.26 The design of this study did not allow us to distinguish between these 2 possibilities. The difference observed on beta-blocker agents, which were more prescribed in individuals who later developed PD and DLB compared to AD or healthy controls, confirms previous similar results and may be explained by their use to relieve tremor.27 The lower risk of developing PD with the use of Beta-2-adrenoreceptors and the higher risk with using beta-blocker agents may also be consistent with the current assumption that β2-adrenoreceptor is a regulator of the α-synuclein gene (SNCA).28 Benzodiazepine's usage have been repeatedly linked to a higher risk of dementia,29 and benzodiazepines were indeed more prescribed in the years preceding with all 3 diseases. Of note, all 3 diseases were also associated with increased rates of anxiety and sleep disorders. As above, the design of the study did not allow us to distinguish between a direct effect of benzodiazepines on the risk of dementia or the consequence of the need to treat prodromal anxiety. Our observed association of benzodiazepines with PD and DLB suggests that this association is not specific to AD.
More surprisingly, we found a lower prescription of statins in the years before the diagnosis of PD compared to dementia-related disorders (AD and DLB). These differences remained significant when adjusting for potential confounding variables, such as APOE status, body mass index, education level, and Townsend deprivation status, and in the replication (UKB) dataset. This observation could have several explanations. First, because statins are prescribed in secondary prevention of cardiovascular diseases, it may be the result of higher vascular comorbidities in the AD and DLB groups.30 However, lipid lowering therapy intake was similar between AD and healthy controls. Moreover, we observed that antihypertensives were similarly prescribed in the 3 diseases, which suggests that the 3 populations may have similar vascular risk factors. Second, AD participants were differentiated from vascular dementia in the UKB. Another explanation would be that subjects treated with lipid lowering therapies are at lower risk to develop PD. Indeed, higher cholesterol levels have been associated with a lower risk of PD,31, 32 including a recent mendelian randomization analysis suggesting that this association could be causal.33 Finally, lipid lowering agents may have a protective effect on PD, as supported by a study showing that the Mediterranean diet adherence is associated with a reduced risk of prodromal PD,34 even though similar studies also exist in AD.35 A recent randomized controlled trial of simvastatin for delay of disease progression in PD was negative,36 indicating that the association might not be causal. Another assumption would be that PD induces a global hypermetabolism and thus fat burning, which could indirectly lower cholesterol levels and thus a lower use of statins. The mechanisms by which lipid lowering therapies may be protective against PD remains to be understood.
Interestingly, the increased usage of statins prior the diagnosis of dementia in the whole population (i.e., AD or DLB) was also found when restricting the analysis in the PD population. Indeed, we found that patients with PD who developed dementia within 5 years used more anti-cholesterol drugs at the pre-PD stage than patients not developing dementia. It may argue toward a specific role of statins in the development of dementia.
Finally, we also identify other prescriptions associated with future development of dementia in the PD population (drugs against constipation and depression). Using past prescriptions of the GP may be useful in the future to design approaches to distinguish patients with PD who will develop dementia shortly after their first initial PD diagnosis.
This study is, to our knowledge, the largest addressing on the same database as the prodromal phase of the 3 neurodegenerative diseases allowing to compare their clinical profile and treatment prescriptions as observed by GPs. The observations of increased non-motor, non-cognitive prodrome in patients with DLB might help GPs who encounter these symptoms in their clinics. Indeed, many are facing challenges of knowing when to refer for subspecialist evaluations, especially in underserved areas. Another notable strength of this study is the replication on the UKB database, with consistent results obtained for the 2 analyses.
Our work has also several limitations. First, in the THIN database, the diagnosis of neurodegenerative diseases was made by GPs, which may result in a delayed diagnosis, explaining why we can observe disease-specific symptoms before their diagnosis. However, most patients have likely been seen by a specialist because previous studies showed that it was the case for 78% of patients with a PD diagnosis.37 Initial diagnosis of probable PD was later confirmed in 83% cases.37 In addition, we used ICD codes, which cannot capture disease severity, which might under-detect soft clinical signs. However, the size of the database offers the opportunity to start progressing our understanding of prodromal risk factors of DLB in France and the United Kingdom. Conversely, in the UKB database, diseases’ records came from hospitalization or death registers, which may be bias toward more severe disease forms. Second, the duration of the study is long. It represents one of the major strengths of this work, because it enables study of possible associations several years before the first diagnosis. However, over that time, diagnostic and prescribing behaviors of generalists have evolved, making the overall cohort heterogeneous in their ascertainment. Third, the frontiers of each complex neurodegenerative disease may not be defined perfectly, and their distinction is a simplification with respect to disorders heterogeneity38 and distinction between developing a DLB or co-existence of PD and AD might be difficult to do in clinical practice. However, the differences observed in the prodromal stage of patients with DLB, show that DLB is not simply the addition of the symptoms of AD and PD at the GP level. Finally, in the THIN analysis, controls were matched with the AD population, which was older and had a higher proportion of female patients than the PD and DLB cohorts, and we may therefore have underestimated associations particularly in the younger PD population. Patients with AD in the UKB might also not be representative of the general patient population as they are still relatively young.
Conclusion
Our results highlight the long-term clinical prodromal phase of neurodegenerative diseases, and therefore could enable more efficient primary and secondary prevention measures. This work also represents one of the first steps to understand early prodromal symptoms of DLB, helping GPs to better detect, and refer to specialists, from the first symptoms of the disease. To the best of our knowledge, this is the first study showing that there may be a differential role of lipid lowering therapies in the prodromal phase of neurodegenerative diseases. Further studies are needed to better understand the relationship among cholesterol levels, the risk of neurodegenerative diseases, and the risk of developing dementia after a PD diagnosis.
Author Contributions
T.N., B.C.-D., A.S., J.-C.C., and S.D. contributed to the conception and design of the study. T.N., B.C.-D., F.M., L.G., A.D.-B., A.S., J.-C.C., and S.D. contributed to the acquisition and analysis of data. T.N., B.C.-D., A.D.-B., F.M., R.C., L.G., B.L., N.V., A.S., S.D., and J.-C.C. contributed to drafting the text or preparing the figures.
Acknowledgments
The research leading to these results has received funding from the joint program in neurodegenerative diseases (JPND) ANR-21-JPW2-0002-01 (LeMeReND) and the program “Investissements d'avenir” ANR-10-IAIHU-06. This work was funded in part by the French government under management of Agence Nationale de la Recherche as part of the “Investissements d'avenir” program, reference ANR-19-P3IA-0001 (PRAIRIE 3IA Institute). B.C.D. is supported by a CJ Martin Fellowship (NHMRC, APP1161356) and the CNRS.
Potential Conflicts of Interest
F.M., L.G., and B.E. are full time employees of Cegedim.
Open Research
Data Availability Statement
The data used in the preparation of the article are available from the Cegedim company upon reasonable request ([email protected]).