Neuropathological Insights into Unexpected Cognitive Decline in Epilepsy
A.J.B. and J.-A.W. contributed equally.
Abstract
Objective
Some patients unexpectedly display an unfavorable cognitive course after epilepsy surgery subsequent to any direct cognitive sequelae of the surgical treatment. Therefore, we conducted in-depth neuropathological examinations of resective specimens from corresponding patients to provide insights as to the underlying disease processes.
Methods
In this study, cases with significant cognitive deterioration following a previous postoperative assessment were extracted from the neuropsychological database of a longstanding epilepsy surgical program. An extensive reanalysis of available specimens was performed using current, state-of-the-art neuropathological examinations. Patients without cognitive deterioration but matched in regard to basic pathologies served as controls.
Results
Among the 355 operated patients who had undergone more than one postoperative neuropsychological examination, 30 (8%) showed significant cognitive decline in the period after surgery. Of the 24 patients with available specimens, 71% displayed further neuropathological changes in addition to the typical spectrum (ie, hippocampal sclerosis, focal cortical dysplasias, vascular lesions, and low-grade tumors), indicating (1) a secondary, putatively epilepsy-independent neurodegenerative disease process; (2) limbic inflammation; or (3) the enigmatic pathology pattern of "hippocampal gliosis" without segmental neurodegeneration. In the controls, the matched individual principal epilepsy-associated pathologies were not found in combination with the secondary pathology patterns of the study group.
Interpretation
Our findings indicate that patients who unexpectedly displayed unfavorable cognitive development beyond any direct surgical effects show rare and very particular pathogenetic causes or parallel, presumably independent, neurodegenerative alterations. A multicenter collection of such cases would be appreciated to discern presurgical biomarkers that help with surgical decision-making. ANN NEUROL 2023;93:536–550
Introduction
Epilepsy surgery has become the therapy of choice for pharmacoresistant patients with focal epilepsy.1 The majority of epilepsy surgical interventions involve temporal lobe structures (temporal lobe epilepsy [TLE]). The most common neuropathological diagnoses include hippocampal sclerosis (HS)2 and highly differentiated, typically glioneuronal tumors (gangliogliomas, “long-term epilepsy-associated tumors” [LEATs]),3-5 as well as focal cortical dysplasias (FCDs).6, 7
The chance of obtaining seizure freedom after epilepsy surgery is accompanied by a risk of postoperative neuropsychological sequelae, for example, episodic memory decline after temporal lobe surgery.1, 8-10 Major determinants of the postsurgical cognitive outcome are the type and quality of surgery (extent, side, site, degree of selectivity, collateral damage, complications),11-13 the functional integrity of resected and surgically affected tissues,14 individual reserve capacities (ie, functional integrity of the remnant brain or homologue contralateral structures),15 and the degree of functional plasticity,16 postsurgical control of epileptic activity,17 and changes in antiseizure medication18 (for review, see Vakharia et al1).
Whereas most cognitive outcome studies have limited observation intervals with an emphasis on the first year after surgery, more and more studies address the neuropsychological longer term outcome.19 Most cohort studies with varying observation intervals of up to 22 years after epilepsy surgery have reported a stable or even improved cognitive status.20-22 Seizure freedom is a relevant factor for a favorable, neuropsychological longer term outcome.22 More selective individually tailored resections were associated with an improved longer-term memory outcome after epilepsy surgery in childhood.21 In a large study from our department, a late significant cognitive decline from the 1-year to the longer term follow-up (5–22 years after temporal lobe surgery) was rare, ranging from 0 to 7% in seizure-free patients versus 11 to 14% in patients with recurrent seizures.22
Evidently, a small subset of patients experience a significant cognitive decline following surgery for TLE, independent of and in addition to the eventual cognitive sequelae of the surgical treatment. Who are these patients, and what are the underlying causes? Considering these precedents, we hypothesized that a systematic neuropathological analysis of resective specimens of TLE patients with unfavorable cognitive development following epilepsy surgery will provide novel insights into relevant pathological parameters. To this end, we selected a large retrospective epilepsy-surgical cohort for cases with an unexpected significant cognitive decline and carried out a systematic in-depth neuropathological analysis. A patient group with the same primary pathologies but stable or even recovered cognitive functions in the period after epilepsy surgery served as controls.
Patients and Methods
Patients
The starting point for this retrospective study at the level 4 epilepsy center in Bonn was to perform a database search of all patients with at least 2 neuropsychological reassessments following epilepsy surgery. First, we searched for the terms “deteriorate*/decline*” in all postoperative neuropsychological reports. The study focused on cognitive decline from one postsurgical assessment to subsequent postoperative testing. Pre- to postsurgical changes were not of interest. Next, we performed a screening of the individual neuropsychological reports with subsequent elimination of irrelevant cases (eg, deterioration in mood and not cognition, or subjective cognitive decline with no objective confirmation). Finally, by thoroughly inspecting postsurgical neuropsychological test performances, we ensured the major inclusion criterion: a significant cognitive decline (according to reliable change indices [RCIs]) in at least one domain (primarily verbal or figural episodic memory, but also executive functions) in the period following surgery (cf Fig S1). Further criteria included age of >18 years at the last follow-up assessment and, at a minimum, the availability of neuropsychological data on verbal and figural episodic memory. Patients with manifest autoimmune encephalitis/limbic encephalitis (LE) according to consensus criteria were not included, because manifest LE is associated with cognitive restrictions and per se a contraindication for epilepsy surgery.23-25 For neuropathological studies with respect to secondary pathology patterns in addition to the primary epilepsy-associated pathological alterations, a control group of patients with stable or recovered cognitive functions in the relevant period following epilepsy surgery was assembled according to the most relevant parameters in the epilepsy disease context, including primary (epilepsy-associated) pathological diagnosis and focus localization determining surgical type/target zone (frontal vs TLE surgery), taking into consideration an optimal approximation of age at seizure onset and age at surgery. The other inclusion criteria were identical.
Informed and written consent was obtained from all patients for additional histopathological studies. Ethical approval for experimental studies of biopsy samples was obtained by the local ethics committee under 007/08. All procedures were conducted in accordance with the Declaration of Helsinki.
Neuropathological Analyses
Surgical specimens were fixed in formaldehyde overnight, embedded in paraffin, and further processed by neuropathological standard procedures as previously described.26, 27 The neuropathological workup was performed by an experienced neuropathologist (A.J.B.) and included stainings with hematoxylin and eosin, as well as immunohistochemical analyses with antibodies against NeuN (Millipore, Billerica, MA), glial fibrillary acidic protein (Agilent/Dako, Glostrup, Denmark), phosphotau (pTau; AT8; Thermo Fisher Scientific, Waltham, MA), amyloid β (Aβ; Anti-Amyloid-β Monoclonal Antibody 4G8; Roboscreen Diagnostic, Leipzig, Germany), microtubule-associated protein 2 (Sigma-Aldrich, St Louis, MO), CD3 (LN10; Leica, Wetzlar, Germany), CD8 (C8; Dako, Glostrup, Denmark), CD20 (L-26, Dako), CD45 (2,811/PD7-26, Dako), CD68 (KP-1, Dako), HLA-DR (CR3-43, Dako), and Syndecan (B-B4; ImmuQuest Seamer, North Yorkshire, UK).
Neuropsychological Assessments
Standardized neuropsychological examinations were conducted as part of the comprehensive presurgical workup28 and as outcome assessments after epilepsy surgery. The core test battery focused on frontal and temporal lobe functions, that is, attention and executive functions and material-specific episodic memory functions, respectively.29
Verbal memory performance was assessed using the Verbaler Lern- und Merkfähigkeitstest (VLMT),30 a modified German version of the Rey Auditory Verbal Learning Test.31 The VLMT is the most frequently employed verbal learning and memory test at epilepsy centers in German-speaking countries.29 The test requires serial learning and immediate recall of 15 words in 5 consecutive learning trials and free recall after distraction, as well as free recall and recognition of the target words after a 30-minute delay. The main parameters considered were learning performance (total number of words learned in 5 trials) and delayed free recall performance. The VLMT has been shown to be sensitive to left temporal lobe dysfunction, left mesiotemporal pathology, and left-sided temporal lobe surgery.32-36
Nonverbal figural memory was assessed using the revised version of the Diagnosticum für Cerebralschädigung (DCS-R).37 The test requires learning and reconstructing 9 abstract designs in 5 consecutive trials. After a retention interval of 30 minutes, recognition memory is tested. The main parameters considered were the number of correctly learned designs over 5 learning trials, as well as the final learning performance. The DCS-R is sensitive to right temporal lobe dysfunction, to right mesiotemporal pathology, and also to right-sided temporal lobe surgery.37-40
To minimize practice effects, parallel versions of the memory tests were employed at the postoperative assessments.
The assessment of attention and executive functions varied over the years and included tests on psychomotor speed (Trail-Making Test A),41 attention shifting (Trail-Making Test B),41 response inhibition (Cerebraler Insuffizienz Test),42 visual planning (maze tests),43 phonemic fluency (subtest of the Leistungs-Prüf-System),44 and working memory (subtest of the revised version of the Hamburg-Wechsler-Intelligenztest für Erwachsene).45 EpiTrack was introduced in 2005 and identifies and merges major aspects of the aforementioned tests into a single score for attention and executive function.46
Statistically significant intraindividual cognitive decline was determined using practice-corrected 90% RCIs based on test–retest data of 81 to 144 healthy controls. RCIs require the calculation of a change score confidence interval and practice effects due to repeated assessment.47 To calculate the change score confidence interval, the following formula was applied: critical difference = ±1.64 × standard error of difference. Practice effects are reflected by the mean changes from the first to the second assessment. To finally obtain a practice-corrected RCI, the lower and upper border of the calculated change score confidence interval is adjusted according these mean changes. A change score beneath the lower border of the RCI or above its upper border indicates a statistically significant intraindividual change.
Statistical Analyses
Descriptive statistics (mean [M] and standard deviation [SD]) were performed with SPSS Statistics 24 (IBM, Armonk, NY).
Results
Neuropsychology-Based Patient Cohort Establishment
The neuropsychological database search revealed n = 355 cases with at least 2 cognitive assessments following epilepsy surgery. Next, we searched for the terms “deteriorate*/decline*” in the neuropsychological reports, which resulted in n = 132 of the 355 cases. A detailed further screening of the individual neuropsychological reports to eliminate irrelevant cases left n = 69 cases. By thoroughly inspecting the postsurgical neuropsychological courses to ensure significant cognitive decline (as compared to a previous postsurgical assessment), we identified n = 30 relevant cases. Resective specimens that allowed for a systematic, in-depth neuropathological examination were available for 24 (80%) of these patients.
Clinical Characteristics
The final sample was comprised of 24 patients with unexpected cognitive decline who had undergone epilepsy surgery in the years between 1988 and 2019 (Table 1). The age at epilepsy onset ranged from 2 to 50 years (M = 16.5, SD = 11.6), and the age at surgery ranged from 15 to 66 years (M = 34.7, SD = 14.5). The vast majority of patients (88%) underwent temporal lobe surgery (n = 21). Three frontal lobe surgeries were performed, one combined with multiple subpial transections. Furthermore, 14 patients (58%) were operated on within the left hemisphere, and 10 patients (42%) were operated on within the right one.
| No. | Age at Epilepsy Onset, yr | Age at Surgery, yr | Type of Surgery | Year of Surgery |
|---|---|---|---|---|
| 1 | 19 | 42 | Right SAH | 2012 |
| 2 | 35 | 37 | Left SAH | 2011 |
| 3 | 16 | 18 | Right LE + AH | 1994 |
| 4 | 23 | 34 | Left SAH | 2019 |
| 5 | 30 | 34 | Right SAH | 2005 |
| 6 | 8 | 54 | Left SAH | 2005 |
| 7 | 18 | 66 | Right SAH | 2002 |
| 8 | 9 | 19 | Right SAH | 1995 |
| 9 | 6 | 31 | Left SAH | 1995 |
| 10 | 23 | 25 | Right ATLR | 1992 |
| 11 | 50 | 58 | Right SAH | 2004 |
| 12 | 2 | 22 | Left frontal LE | 2004 |
| 13 | 7 | 18 | Left ATLR | 1998 |
| 14 | 16 | 45 | Right frontal partial resection + MST | 1995 |
| 15 | 4 | 20 | Left temporal LE without hippocampus | 1992 |
| 16 | 13 | 19 | Right frontal pole resection | 1991 |
| 17 | 7 | 52 | Left ATLR | 1991 |
| 18 | 4 | 42 | Left temporal pole resection + AH | 2016 |
| 19 | 26 | 47 | Left SAH | 2015 |
| 20 | 22 | 50 | Left temporal partial resection | 2008 |
| 21 | 6 | 26 | Left SAH | 2008 |
| 22 | 27 | 32 | Left temporal LE | 1996 |
| 23 | 14 | 27 | Left temporal resections | 1991 |
| 24 | 11 | 15 | Right ATLR | 1988 |
- Abbreviation: AH = amygdalohippocampectomy; ATLR = anterior temporal lobe resection; LE = lesionectomy; MST = multiple subpial transections; SAH = selective AH.
The medical history (Table S1) indicated a psychiatric comorbidity in 7 patients (29%), including borderline personality disorder, depression, anxiety disorder, and a history of psychosis. Perinatal hypoxia or asphyxia was reported in 2 patients (8%), a forceps delivery in 2 further patients (8%), a spina bifida in 1 (4%), and meningitis as an infant in 2 other cases (8%). Furthermore, 1 patient (4%) had neurofibromatosis type I and a chiasma glioma, 1 (4%) had Klinefelter syndrome, and 1 (4%) had head trauma.
The control sample included 17 patients without cognitive decline in the time following epilepsy surgery in the years between 1989 and 2009. The age at epilepsy onset ranged from 1 to 35 years (M = 10.6, SD = 10.9), and the age at surgery ranged from 13 to 51 years (M = 27.2, SD = 12.3). Temporal lobe surgeries were performed in 82% and frontal lobe surgeries in 18% of the control cohort. Left- versus right-sided surgeries were performed in 59% versus 41% of the patients, respectively.
Cognitive Decline
The number of postoperative neuropsychological examinations ranged from 2 to 5 assessments (see Table S1 and Fig S1). The final cognitive assessments were conducted 11 to 255 months after the surgical procedure. Objectively assessed deterioration in cognition was mostly observed once during the postsurgical course and, predominantly, at the latest postoperative assessment (see Table S1 and Fig S1). Five patients showed repeated cognitive decline, 4 of them displayed repeated occurrences twice, and 1 even 3 times. The most frequently affected cognitive domain was verbal memory (96%), followed by figural memory (33%) and executive functions (25% including 1 patient with global decline; see Table S1 and Fig S1).
In 1 patient, brain imaging indicated the development of atherosclerotic macro- and microangiopathy following surgery; in another patient, global and predominantly right hippocampal atrophy plus atherosclerotic microangiopathy was found. In a further case, a progredient contralateral temporal lesion was described, and in another patient, oligoclonal bands were registered in the time after surgery. In all 5 patients with tumors, no evidence of tumor recurrence was found that could have been responsible for the cognitive decline and no radiotherapy was given in addition to the resective treatment.
Potential Contributing Factors in Regard to Cognitive Decline
A major potential contributing factor with regard to cognitive decline is the postsurgical seizure status (see Table S1). Only 4 patients (17%) have been continuously seizure-free, although 2 further patients (8%) became seizure-free after early postsurgical seizures. A late relapse was reported in 10 patients (42%), 3 of whom had a single paroxysmal event, whereas a new semiology was discussed in 4 patients (1 of them with a contralateral seizure onset). Among the remaining patients with ongoing seizures after epilepsy surgery, 2 displayed an increased late seizure frequency, 2 showed a late status epilepticus (1 with convulsive status), and 1 displayed declining seizure frequency.
In 1 patient (4%), the introduction of antiseizure medications with adverse neuropsychological side effect profiles (here: topiramate and clobazam) might have contributed to the observed cognitive decline.
Further potential impact factors included de novo head trauma, de novo depression, and pregnancy in 1 patient each (see Table S1).
Neuropathological Spectrum
The neuropathological specimens of all patients included in the present series (n = 24) revealed primary diagnoses that reflect the typical spectrum of chronic focal epilepsy-associated pathology. Those included (“compatible with”) HS (n = 12), low-grade LEATs (comprising gangliogliomas [World Health Organization (WHO) grade I, n = 3], low-grade astrocytoma [WHO grade II, n = 1], and polymorphous low-grade neuroepithelial tumor [n = 1]), cortical dysplasia (type IIa, n = 1), a vascular lesion fulfilling morphological criteria of cavernoma (n = 1), hippocampi lacking segmental neurodegeneration (n = 3), and extratemporal brain tissue with “diffuse reactive gliosis” (n = 2; Table 2). Notably, 88% (n = 21) of biopsy specimens were from temporal and 13% (n = 3) from frontal localization. For both localizations, no preponderance for either extensive or minimal resections was detected.
| No. | Primary pathology | Localization |
|---|---|---|
| In secondary pathology group "hippocampal gliosis only" | ||
| 1 | No segmental neurodegeneration | Temporal |
| 2 | No segmental neurodegeneration | Temporal |
| 3 | No segmental neurodegeneration | Temporal |
| In secondary pathology group "LE-like" | ||
| 4 | HS | Temporal |
| 5 | HS | Temporal |
| 6 | HS | Temporal |
| 7 | HS | Temporal |
| 8 | Hippocampal fragments, compatible with HS | Temporal |
| 9 | HS | Temporal |
| 10 | HS | Temporal |
| In secondary pathology group "neurodegenerative" | ||
| 11 | HS | Temporal |
| 12 | FCD type IIa | Frontal |
| 13 | Astrocytoma (WHO grade II) | Temporal |
| 14 | Gray & white matter with diffuse reactive astrogliosis | Frontal |
| 15 | Ganglioglioma (WHO grade I) | Temporal |
| 16 | Gray & white matter with diffuse reactive astrogliosis | Frontal |
| 17 | HS | Temporal |
| No secondary pathology | ||
| 18 | HS | Temporal |
| 19 | HS | Temporal |
| 20 | Ganglioglioma (WHO grade I) | Temporal |
| 21 | HS | Temporal |
| 22 | Cavernoma | Temporal |
| 23 | Ganglioglioma (WHO grade I) | Temporal |
| 24 | PLNTY (WHO grade I) | Temporal |
- Abbreviation: FCD = focal cortical dysplasia; HS = hippocampal sclerosis; LE = limbic encephalitis; PLNTY = polymorphous low-grade neuroepithelial tumor; WHO = World Health Organization.
Intriguingly, the majority of biopsy specimens (71%) revealed pathology patterns that are unexpected within the context of epilepsy surgery, including "hippocampal gliosis only" (n = 3), reflecting the "no HS, gliosis only" International League against Epilepsy classification2; LE-like infiltrates (n = 7); and neurodegenerative alterations (n = 7). No significant differences were noted with respect to the age distribution among the 4 different groups of secondary pathology/no secondary pathology. Whereas “gliosis only” hippocampi lacked the HS-typical segmental neuronal cell loss and fibrillary astrogliotic pattern pronounced in the damaged areas (Fig 1A–C), hippocampi with LE-like characteristic alterations revealed the damage pattern of HS plus varying lymphocytic infiltrates. In addition to all patients in this LE pattern group harboring interspersed or clustered hippocampal T cells (see Fig 1D–H), only 1 resective specimen (Patient 10) revealed additional significant B-lymphoid and plasma cellular infiltrates (Table 3). Seropositivity was encountered in only a single patient of those with LE-characteristic alterations (Patient 4, anti-Yo autoantibody). However, this positivity was only transient (in 2015) and not present at the time of epilepsy surgery (2019). None of the patients harboring LE-like findings in the surgical biopsies fulfilled criteria of manifest autoimmune LE.
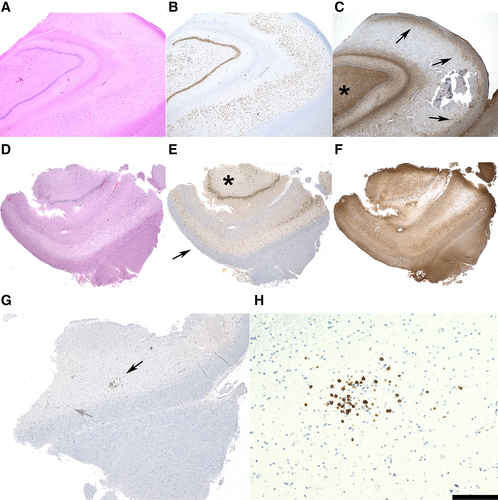
| No. | CD3 | CD8 | CD20 | Syndecan |
|---|---|---|---|---|
| 4 | +++ | +++ | − | − |
| 5 | ++ | ++ | − | − |
| 6 | ++ | ++ | − | − |
| 7 | ++ | ++ | − | − |
| 8 | ++ | ++ | − | − |
| 9 | ++ | ++ | − | − |
| 10 | +++ | +++ | ++ | ++ |
- Abbreviation: − = absent; + = weak; ++ = intermediate; +++ = strong infiltrates.
Distinct neurodegenerative alterations reflected by Aβ or tau pathology, in addition to primary focal epilepsy-associated pathology patterns, were observed in biopsy specimens of n = 7 patients of the present series. Aβ was mainly present in frontal specimens (n = 3; extrafrontal, n = 1), whereas pTau was detected in exclusively temporal specimens (n = 3). The presence of AT8-positive pTau structures was associated with HS (n = 2) and was also present in the preexisting temporal cortex of a ganglioglioma (n = 1). Aβ pathology was associated with dysplastic (n = 1) and neoplastic (n = 1) lesions as well as with unspecific changes in the sense of “diffuse astrogliosis” (n = 2). The Aβ deposits and pTau-positive neurofibrillary tangle recapitulating structures were virtually exclusive in these specimens (Table 4).
| No. | Neurodegenerative features |
|---|---|
| 11 | Neurofibrillary tangles |
| 12 | Patchy Aβ deposits |
| 13 | Patchy Aβ deposits |
| 14 | Aβ plaquelike structures |
| 15 | Neurofibrillary tangles |
| 16 | Aβ plaquelike structures |
| 17 | Neurofibrillary tangles |
- Abbreviation: Aβ = amyloid β.
AT8-positive, pTau-containing neurons were present in hippocampal as well as extrahippocampal biopsy tissue. In neocortical area upper layers, preferentially layer II neurons were AT8-positive (Fig 2A–E). Aβ deposits were present in frontal (n = 3) and temporal (n = 1) locations. They comprised small dotted extracellular positivity patterns, as well as structures recapitulating the morphological architecture of amyloid plaques (see Fig 2F–H).
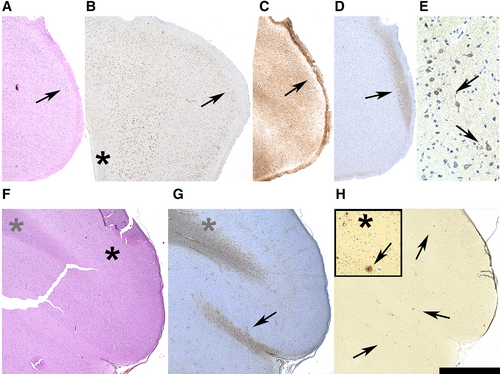
The spectrum of primary pathologies in resective specimens that lacked other—inflammatory or specific neurodegenerative—alterations was remarkably diverse, comprising HS (n = 3), LEATs (n = 3), and cavernoma (n = 1; see Table 2).
A cohort matched as closely as possible with respect to individual parameters including a corresponding principal pathology spectrum and identical surgical interventions with the study group but with stable cognitive findings or even recovery in the postoperative period served as control (Tables 5 and 6). None of these control patients revealed the combination of the primary epilepsy-associated pathology in concert with the specific secondary pathology pattern observed in the primary study cohort.
| No. | Age at Epilepsy Onset, yr | Age at Surgery, yr | Type of Surgery | Year of Surgery |
|---|---|---|---|---|
| 1 | 35 | 40 | Right ATLR | 1992 |
| 2 | 1 | 26 | Left ATLR | 1991 |
| 3 | 5 | 19 | Left ATLR | 1991 |
| 4 | 21 | 34 | Left SAH | 1998 |
| 5 | 2 | 26 | Right SAH | 1993 |
| 6 | 14 | 51 | Left SAH | 1994 |
| 7 | 1 | 30 | Left SAH | 1997 |
| 8 | 2 | 19 | Right ATLR | 1989 |
| 9 | 12 | 17 | Right SAH | 1999 |
| 10 | 14 | 22 | Right ATLR | 1992 |
| 11 | 35 | 51 | Left SAH | 1995 |
| 12 | 6 | 16 | Left frontal LE | 2009 |
| 13 | 13 | 13 | Right temporal LE | 1994 |
| 14 | 9 | 36 | Right frontal partial resection | 1989 |
| 15 | 5 | 14 | Left temporal LE including hippocampus | 1993 |
| 16 | 4 | 14 | Left frontal LE + MST | 1997 |
| 17 | 1 | 35 | Left SAH | 1994 |
- Abbreviation: ATLR = anterior temporal lobe resection; LE = lesionectomy; MST = multiple subpial transections; SAH = selective amygdalohippocampectomy.
| No. | Primary Pathology | Localization |
|---|---|---|
| No secondary pathology pattern "hippocampal gliosis only" | ||
| 1 | No segmental neurodegeneration | Temporal |
| 2 | No segmental neurodegeneration | Temporal |
| 3 | No segmental neurodegeneration | Temporal |
| No secondary pathology pattern "LE-like" | ||
| 4 | HS | Temporal |
| 5 | HS | Temporal |
| 6 | HS | Temporal |
| 7 | HS | Temporal |
| 8 | Hippocampal fragments, compatible with HS | Temporal |
| 9 | HS | Temporal |
| 10 | HS | Temporal |
| No secondary pathology pattern "neurodegenerative" | ||
| 11 | HS | Temporal |
| 12 | FCD type IIa | Frontal |
| 13 | Ganglioglioma (WHO grade I) | Temporal |
| 14 | Gray & white matter with diffuse reactive astrogliosis | Frontal |
| 15 | Ganglioglioma (WHO grade I) | Temporal |
| 16 | Gray & white matter with diffuse reactive astrogliosis | Frontal |
| 17 | HS | Temporal |
- a Patients included in this control group shared the primary pathology with the study group, but in contrast lack (1) corresponding neuropsychological decline and (2) the combinatory pathology patterns observed in the study cohort affected by neuropsychological parameter decline.
- Abbreviation: FCD = focal cortical dysplasia; HS = hippocampal sclerosis; LE = limbic encephalitis; WHO = World Health Organization.
Discussion
Our study addressed the issue of unexpected cognitive decline in epilepsy and took advantage of the unique opportunity that epilepsy surgery provides for gaining insight into what may be the underlying pathology. We conducted state-of-the-art neuropathological reexaminations of specimens from patients who unexpectedly had an unfavorable cognitive course following epilepsy surgery and subsequent to any (direct) cognitive sequelae of the surgical treatment.
Neuropsychology
Eight percent of all 355 patients with at least 2 neuropsychological assessments after epilepsy surgery showed a relevant cognitive decline from one postoperative follow-up examination to a subsequent evaluation.
The most frequently affected cognitive domain by far was verbal memory (96%), followed by figural memory (33%) and executive functions (25%). Repeated cognitive declines in the time after surgery were observed in 5 of the 24 patients (21%).
The medical reports of only 3 of the final 24 patients reported de novo structural brain alterations including atrophy, macro- and microangiopathy, or a progredient contralateral temporal lesion. In 1 patient, oligoclonal bands were found.
We performed an in-depth inspection of the patients' medical reports to identify any potential confounders with regard to the observed cognitive decline. A seizure relapse was seen in 42% of the patients, and a worsening of the seizure status was found in an additional 21%. Only 25% achieved early (17%) or late (8%) seizure freedom, which has been identified as a relevant factor for a favorable cognitive longer term outcome.22 In 1 patient, antiseizure medication with known adverse cognitive profiles (topiramate48, 49 and clobazam50) was introduced. Other potentially relevant factors were single cases with a de novo head trauma, de novo depression, and pregnancy.51 All of these factors may have contributed to (in varying degrees) the observed intraindividual cognitive decline.
Neuropathology
With respect to the macro- and microscopic neuropathological findings in resective tissue samples of the present series of patients with unfavorable cognitive development in the postsurgical course, it is striking that the extent of the resection that was obtained, particularly in temporal localization, from which tissue of large (lobectomies/lesionectomies plus amygdalohippocampectomy) or only minimal resections (selective amygdalohippocampectomies), did not lead to a robust overrepresentation of either group in the present patient cohort. This observation may argue that factors other than the extent of resection in temporal localization are decisive for unfavorable cognitive courses after temporal epilepsy surgery.21 In the vast majority of cases (88%), temporal structures were affected and the major secondary pathology patterns involved inflammatory infiltrates of the hippocampal formation given as (1) signs reflecting encephalitis as well as (2) “no HS, gliosis only.”2, 52
Notably, all patients, other than 2 individuals with a frontal seizure focus as well as 3 patients with hippocampal “gliosis only,” had a specific diagnosis that is typical in the context of epilepsy surgical specimens. These diagnoses are HS, dysplasia, LEAT, and vascular malformation. For Patients 14 and 16 with frontal seizure foci, it remains an open question whether “diffuse reactive astrogliosis” represents an unspecific finding or the extrahippocampal surrogate of “gliosis only.”53 In the “gliosis only” biopsies, a robust cellular astrogliosis component is encountered that is typically absent in HS, with a more fibrillary gliosis pattern in segments with strong neurodegeneration.53 Additional pathology patterns in the present resective specimens of patients were characterized to recapitulate features of adaptive inflammation termed “LE-like,” as well as “neurodegenerative,” given as Aβ deposits or pTau-positive neuropil threads or neurofibrillary tangles. The observation that these pathological findings were virtually exclusive may suggest that each individual alteration, that is, adaptive immunity, Aβ deposits, and pTau positivity, per se and independently, can be associated with an unfavorable cognitive development. Aβ deposits and pTau positivity do not occur independently of a primary epileptogenic lesion, again assuming “diffuse reactive astrogliosis” to be an extrahippocampal surrogate of “gliosis only.” Thus, these alterations represent disease modifiers with respect to network function rather than primary epileptogenic alterations. In contrast, LE-like and “gliosis only” alterations may constitute seizure foci and harm neuronal network function,54, 55 manifesting as poor neuropsychological performance.
Gliosis Only
We recently reported on magnetic resonance imaging of this lesion pattern in patients with pharmacoresistant TLE and hippocampal specimens lacking significant segmental neuronal cell loss,53 which reflects the category “no HS, gliosis only” of the corresponding classification of the International League against Epilepsy.2 Our own unpublished data suggest that approximately 20% of the patients with hippocampal seizure origin lack the damage pattern of HS and correspond to “no HS, gliosis only.” “Gliosis only” putatively due to the diffuse cellular pattern, which lacks focality, might only be poorly controllable with surgery.55 Astrogliosis represents a main substrate of innate immune signaling.56 Tissue manipulation may even stimulate the innate immune signaling. Glia-mediated excitation and inflammation may not only mediate or trigger seizures,52 but be well suited to constitute a sustained condition of neuronal network impairment. This manifests with a lower-than-average postsurgical cognitive recovery, and persistent or even declining neuropsychological performance. The potential molecular mechanisms are manifold and involve increased oxidative stress, impaired astroglial glutamate metabolism, and extracellular matrix remodeling.57-59 Importantly, the present hippocampal specimens with the pattern of “no HS, gliosis only” lacked significant lymphocytic or plasma cellular infiltrates such that the differential diagnosis of LE could be virtually excluded.
LE-like
Decline of cognitive function has been associated with different autoantibodies related to LE, including such targeting molecules at the neuronal surface, GAD65 and so-called onconeuronal antibodies.60-69 In the present study cohort, only patients were included who did not fulfill key diagnostic criteria for autoimmune encephalitis/LE,23, 25 because active autoimmune LE per se is generally regarded as a contraindication for epilepsy surgery and furthermore the neuropsychological sequelae of LE do not meet the features of "unexpected" postsurgical memory decline, the major feature of our study cohort. With respect to Patient 4, anti-Yo was found only once in 2015, and was negative in several follow-up tests. Considering the rather weak link of anti-Yo with LE,70, 71 seronegative results of repetitive autoantibody testing for the time after 2015 in Patient 4, and also clinical criteria incompatible with a definite diagnosis of LE, the patient underwent epilepsy surgery due to pharmacorefractory temporal lobe epilepsy in 2019. Because the patient did not fulfill the criteria for LE, the neuropathological diagnosis was not "compatible with LE" but rather "LE-like."
The LE-like hippocampi showed prominent T cells but only very few B cells or plasma cells, that is, inflammatory components characteristically reported in manifest LE.72 In particular, the neuropathological coincidence of neurodegenerative tissue damage in concert with the invasion of T lymphocytes in hippocampal specimens may be compatible with the presence of an adaptive immune reaction against intracellularly localized target structures as known, for example, for anti-GAD65 and onconeuronal antibodies.60, 73, 74 In LE-like hippocampi, only few, putatively residual cytotoxic T cells are found in hippocampal areas where extensive neurodegeneration has already taken place, and clusters of perineuronally arranged cytotoxic T cells are present at adjacent sites, presumably subject of acutely active inflammatory destruction of neurons (see Fig 1). Importantly, recent data indicate that per se T-cell numbers in TLE biopsy tissue correlate with neuronal cell loss. This may be a feature importantly linked to cognitive performance rather than to seizure activity.75 Extensive destruction of gray matter extending to the hippocampus and limbic structures has been reported for LE patients with anti-GAD65 and other intracellularly located antibody targets.60, 61 These neuropathological substrates may be well compatible with unfavorable postsurgical cognitive outcomes in patients with HS on the basis of LE.24, 67 Our present data, which include patients with extremely poor postsurgical cognitive follow-up parameters, argue for pathomechanisms similar to those in manifest LE as a major determinant in this context. That the majority of patients in this subgroup lack proof of seropositivity for specific autoantibodies is compatible with recently published data on the presence of seropositivity for specific autoantibodies in only a subfraction of patients strongly suspicious for LE.71 Of note, a history of a definitely expired LE is not perceived as a contraindication for epilepsy surgery. An emerging, static lesion generally given by HS secondary to a previous (and now inactive) inflammatory process may be a surgical target for the treatment of epilepsy after the careful clinicoserological confirmation of the expired LE status of the patient.
The lack of corresponding "LE-like" findings in the control group may indicate that the neuropsychological course of patients has a strong potential impact to identify "LE-like" HS patients. These data may emphasize the necessity of intensified presurgical analyses for biomarkers including potentially new autoantibodies in serum and cerebrospinal fluid to determine LE(-like) status features presurgically and, depending on the results, a reconsideration of patient management.71
Neurodegeneration: Aβ- and pTau-Related Pathologies
Neurodegenerative-associated pathology patterns have been debated in the context of TLE, and only recently, a study observed only a low prevalence of characteristic neuropathological patterns.76 Several aspects of patients from the present series affected by Aβ- or pTau-related pathologies appear remarkable. Seizure foci were either frontal or temporal instead of only temporal in all patients of all other pathology subgroups. More than 50% of the patients with such neuropathological changes of the present series had a temporal seizure focus. The main “specific neurodegenerative” feature of these TLE patients were AT8-positive neurofibrillary tangles (3 of 4 patients). In contrast, all patients (3 of 3 patients) with frontal resections revealed Aβ-associated deposits; that is, at an anatomical site where Aβ deposits are often primarily observed in Alzheimer disease (AD).77 Intriguingly, both alterations, AT8-positive neurofibrillary tangles and Aβ plaques, were present adjacent to lesions—including low-grade tumors and dysplasias—in preexisting central nervous system tissue portions. In the present cases, we did not observe expression of Aβ or pTau by dysplastic neuronal elements in low-grade tumors or malformations as demonstrated by others before.78, 79
Several patients at the time of surgery were rather young for the presence of Aβ pathology. This aspect may argue against the presence of a neurodegenerative disorder, including AD or frontotemporal lobar degeneration (FTLD), as a secondary independent disease. Consequently, seizure-associated changes may constitute the preferential pathogenetic scenario. A typical neuropathological substrate of chronic focal epilepsy given by FCD type IIa was only present in 1 of 3 patients with frontal seizure focus and Aβ deposits. In the other 2 affected patients, Aβ deposits co-occurred in concert with reactive astrogliosis. However, it cannot be determined whether the Aβ deposits are consequential in this context, promoting or even causing recurrent seizures and cognitive dysfunction.
With respect not only to the localization, but also to the primary focal epilepsy-associated neuropathological findings, the “neurodegenerative” patient cohort showed the highest degree of heterogeneity of the present series, being even more diverse than the “no secondary pathology” group. Thus, seizure activity itself but not a distinct primary pathology represents the common denominator associated with the observed Aβ- or pTau-related changes observed here. Interestingly, inflammation-associated seizure foci such as antibody-associated LE are not at all encountered here as primary pathology.
pTau-positive neurofibrillary tangles were only observed in temporal localization, including the hippocampal formation as well as layer II neurons, thus reflecting patterns that were recently reported to correlate with cognitive decline in chronic epilepsy patients with temporal lobe resections.80, 81 Our present findings corroborate these observations and are in favor of the role of distinct pTau distribution patterns to elicit network impairment manifesting with severely compromised cognitive performance. Considering the particular distribution pattern, we interpret the present pTau deposits as epilepsy-associated alterations rather than the neuropathological equivalent of, for example, AD or FTLD. However, it must be noted that the available surgical specimens do not allow a systematic staging of Aβ- or pTau-related pathology patterns such as in the AD scheme.82, 83 In cognitively normal subjects, AD-type alterations were described in a ≥40-year age range and Aβ deposition was not observed in the absence of neurofibrillary tangles.84 Given that some of the patients in our present cohort are rather young, seizure activity may be perceived as a pathogenetically promoting factor for neurodegenerative alterations. Follow-up information on the development of an independent neurodegenerative disease would have been particularly interesting for our present patients, but was unfortunately not available.
Several reports are in favor of abundant Aβ deposits under epilepsy conditions, whereby the significance of cognitive impairments was diverse between different studies. Immunohistochemically, the Aβ precursor protein was abundant in temporal lobectomy tissue compared to age-matched controls.85 Other studies described an age-accelerated presence of senile plaques in 10% of the epilepsy cohort, and Aβ plaques in 15% of cases in surgical specimens of patients aged 50 to 65 years without a significant correlation to postsurgical cognitive outcome.80, 86 A large postmortem study observed Aβ plaques in 44% of individuals with chronic epilepsy, but did not find a correlation to premortem cognitive assessments.87
Neurofibrillary tangles reportedly manifested in an age-accelerated pattern of advanced Braak stages (III/IV) in a postmortem series of 40- to 60-year-old pharmacoresistant epilepsy patients compared to age-matched controls.87 A recent study demonstrated increased pTau according to age in temporal lobe resection specimens to be significantly correlated to a decline in verbal skills.80 More subtle hyperphosphorylated tau within the dentate gyrus and subiculum in a younger series with HS was significantly associated with naming score decline 1 year postoperatively.88
No “Secondary” Pathology
This group is mainly comprised of HS and temporal LEATs. We cannot exclude false negative lack of detection of secondary pathology, for example, due to sampling constraints. With respect to HS, it cannot be excluded that distinct coinciding patterns of pathogenetic features, including particular neurodegenerative and network reorganization characteristics, manifest in those patients having an extremely poor postsurgical cognitive outcome. So far, several studies have reported on different correlations of such morphological aspects with memory function (for review, see Tai et al89).
Limitations
This is a retrospective analysis with all associated limitations. Furthermore, not all patients underwent brain imaging at the time when the cognitive decline was detected. Based on a comprehensive in-depth inspection of the medical history of each patient, we identified potentially relevant impact factors that may have also contributed to the unexpected cognitive decline. However, all these additional factors may have contributed (in varying degrees) to the observed intraindividual cognitive decline, but we cannot see that any of the identified factors could fully explain the cognitive decline.
Conclusions
Neuropathological reanalysis of patients displaying unexpected cognitive decline following epilepsy surgery revealed rare and very particular pathogenetic causes, or parallel, presumably independent, neurodegenerative alterations. We found a high incidence of innate or adaptive immune as well as neurodegenerative mechanisms not present in a control series without postsurgical cognitive decline. Therefore, our present major neuropathological findings have several implications. In clinical practice, inflammatory conditions and neurodegenerative changes need to be taken into account in case of an unexpected cognitive decline in epilepsy. Prior to performing epilepsy surgery, repetitive neuropsychological assessments may help to disclose any cognitive dynamics that could indicate an underlying inflammatory pathology. Postsurgically, the neuropathological workup of epileptogenic tissue should systematically take inflammatory and neurodegenerative aspects into account. A multicenter collection of such cases would be appreciated, to discern (presurgical) biomarkers that help with surgical decision-making. In concert with neuropsychological assessments after epilepsy surgery, patients with inflammatory as well as neurodegenerative disease aspects may profit from more tailored postsurgical treatments in the future.
Acknowledgments
Our work is supported by Deutsche Forschungsgemeinschaft (SFB 1089 and FOR2715 to A.J.B.), CONNECT-GENERATE (FKZ01GM1908C to A.J.B.), and the EKFS-Promotionskolleg "NeuroImmunology" by the Else Kröner-Fresenius Foundation (A.R.).
We thank the Bonn Medical Faculty for providing the framework of the Epilepsy-Surgical Biobank. The present work fulfils the first author's obligations for A.R.'s written thesis to obtain the MD degree according to the "Doctoral Degree Regulations" at the University of Bonn Medical Faculty. We thank Dr J. Schramm, a neurosurgeon with long-term contribution to the epilepsy surgery program, for critical comments on the manuscript. We thank K. Wörmann for proofreading and language editing. Open Access funding enabled and organized by Projekt DEAL.
Author Contributions
C.E.E., C.H., A.J.B., A.R., and J.-A.W. contributed to the conception and design of the study. All authors contributed to the acquisition and analysis of data. All authors contributed to drafting the text or preparing the figures.
Potential Conflicts of Interest
Nothing to report.
Open Research
Data Availability Statement
Available data are presented in the tables and in the supplementary material.