Development and treatment of colorectal cancer: Insights from multi-kingdom microbiota
Abstract
Colorectal cancer (CRC) is a multistage and highly heterogeneous malignant disease that mostly occurred in aged people accompanied by microbiota alteration. Emerging evidence has uncovered the role of bacterial microbiota in the initiation and development of CRC. However, the effects of nonbacterial members inhabiting the human body, such as fungi, archaea, and viruses, have been largely ignored. The multi-kingdom microbiota can be altered by dietary exposures and probiotic supplements. Furthermore, the efficacy of antitumor therapeutic strategies, such as radiotherapy, chemotherapy, and immunotherapy, are also closely associated with the alteration of multi-kingdom microbiota. In this review, we describe CRC-associated multi-kingdom microbiota dysbiosis and the role of daily diet on CRC progression through microbiota alteration. We then discuss the impact of microbiota in different CRC therapies and highlight the advances as well as challenges in understanding how multi-kingdom microbiome impacts the outcome of CRC.
1 INTRODUCTION
Colorectal cancer (CRC) is the third most frequently diagnosed cancer and the second leading cause of cancer-related death.1 There were an estimated 149,500 new cases and 52,980 deaths of CRC in the United States according to the most recent reports.2 CRC incidence increases with age, from 68.4 per 100,000 in populations from 55- to 59-year old to 121.4 per 100,000 in people from 65- to 69-year old.3 Notably, aging is currently a trend in most countries, and studies have tried to uncover the relationship between aging and cancer pathogenesis as they have shared hallmarks, including genomic instability, telomere attrition, abnormal proteostasis, heightened inflammation, and increases in cellular senescence.4, 5 Likewise, epigenomic modifications like acetylation and methylation were found to be affected in cancer cells,6, 7 whereas Braf mutation can promote intestinal epithelium unnormal methylation and induce neoplastic conversion during aging.8 Relative telomere length was shortened in CRC tissue compared with normal tissue, which is found in the aging cell as well.9 Mitochondrial function is also affected during aging as well as in cancer development. Recent studies reported the alteration of oxidative metabolic profile and damage of mitochondrial function in the late stage of CRC.10
Gut microbiome is recently acknowledged as a new target in preventing aging, as it plays a critical role in maintaining the integrity of gut barrier and immune homeostasis.11, 12 The abundance of gut microbes differs among young adults to aged people. Firmicutes and Bacteroidetes were enriched in adults, whereas Bacteroidetes and Proteobacteria were dominated in aged people with the deletion of Firmicutes.13-15 On the other side, the relationship between gut microbiota and CRC was also well documented. Conceptually, the manipulation of gut microbiota would be feasible in the treatment of CRC and aging-associated health problems, and diets have been proven to exert viral impacts on microbiota composition and diversity.16
Gut microbiota, which is composed of bacteria, fungi, archaea, and viruses, is highly flexible and can be influenced by various factors, including diet.16 However, most of these studies only focused on bacteria, and few of them have examined the relationship between nonbacterial species and CRC development. In this review, we briefly describe the dysbiosis of multi-kingdom microbiota during CRC development, the interactions of bacteria, nonbacterial gut microbes, and diet in the development of CRC. We also review the relationship between microbiota and different therapies and illustrate the role of diet in the prevention of CRC through gut microbiota modification.
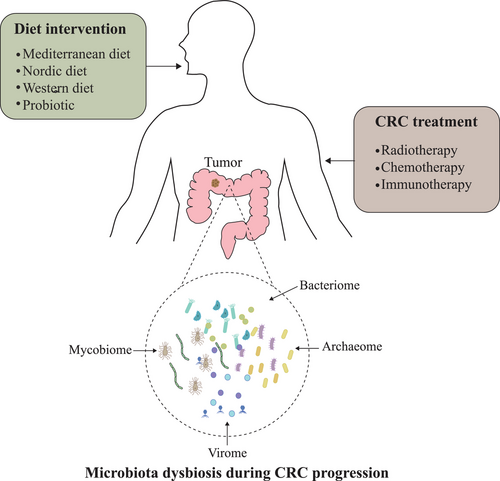
2 MICROBIOTA DYSBIOSIS DURING CRC PROGRESSION
2.1 Four-kingdom microbiome in healthy subjects
The human microbiome was composed of bacteria, fungi, viruses, protozoa, and related genes and metabolites.17, 18 Of those, the bacteria, which is dominated by three major phyla: Firmicutes, Bacteroidetes, and Actinobacteria, has been extensively studied.19 In contrast, fungi only account for approximately 0.2% of microorganisms in the human gut, predominated by three phyla, including Ascomycota, Basidiomycota, and Chytridiomycota.20 Archaea is the single-cell prokaryote with distinct cellular characteristics from either bacterium or eukaryotes, such as the lack of peptidoglycan and d-glycerol esters or fatty acids.21 The major components of human gut archaeome are species from Methanobacteriales and Methanomassiliicoccales.22 The human gut virome includes double-stranded DNA viruses, single-stranded DNA viruses, as well as RNA viruses.23 Particularly, intestinal bacteriophages account for nearly 90% of human virome.24
2.2 Bacterial microbiota dysbiosis in CRC
Disruption of gut bacterial microbiome homeostasis induces chronic inflammation of the GI tract and production of carcinogenic metabolites, which leads to colon damage.25 In studies comparing the composition of gut microbiota between CRC patients and healthy subjects, the abundance of Bacteroides fragilis, Fusobacterium nucleatum (Fn), Parvimonas micra, Porphyromonas asaccharolytica, Prevotella intermedia, Alistipes finegoldii, and Thermanaerovibrio acidaminovorans in CRC patients were increased, which can also be used as diagnostic markers.26, 27 However, the abundance of Roseburia, Clostridium, Faecalibacterium, and Bifidobacterium were significantly reduced.28, 29 It was demonstrated that B. fragilis downregulated miR-149-3p, which has been linked to intestinal inflammation and malignancy.25 FadA adhesin in Fn can help to promote CRC cells proliferation through E-cadherin/β-catenin signaling.30 In addition, Fn can also impact tumor microenvironment recruiting tumor-infiltrating myeloid cells and potentiated CRC metastasis by modulating miR1322/CCL20 axis, miR-1246/92b-3p/27a-3p, CXCL16, and KRT7-AS/KRT7.31-33 Besides, several bacterial species known as probiotics were also absent in CRC patients. The abundance of Akkermansia muciniphila was reduced in dextran sulfate sodium (DSS) treat mice, whereas Lactobacillus reuteri and Clostridium butyricum were reduced in CRC patients.34-36 Detailed information regarding CRC-associated bacterial microbiome alteration reported by previous studies is summarized (Table 1). Taken together, these studies suggested that gut microbiota was significantly altered in CRC compared with healthy control, and certain species (like B. fragilis and F. nucleatum) were commonly changed across studies.
| Bacteria dysbiosis | |||||
|---|---|---|---|---|---|
| Sample size | Sample type | Study design | Microbiota analysis | Main finds comparing cases to controls | References |
| 46 CRC patients/56 healthy subjects | Fecal/gut swab/tissue | Case-control | 16s rRNA | ↑Lactobacillales, ↓Faecalibacterium | 37 |
| Mucosa-adherent microbiota, ↓Bifidobacterium, Faecalibacterium, and Blautia, ↑Fusobacterium, Porphyromonas, Peptostreptococcus, and Mogibacterium | |||||
| 19 CRC patients/20 healthy subjects | Fecal | Case-control | 16s rRNA | ↑Bacteroid, ↓butyrate-producing genera Faecalibacterium, and Roseburia | 28 |
| 11 CRC patients/10 healthy subjects | Fecal | Case-control | 16s rRNA/GC-MS | ↑Akkermansia muciniphila, acetate, and amino acids | 29 |
| 19 CRC patients/12 adenomas patients/19 healthy subjects | Fecal | Case-control | 16s rRNA | ↑Fusobacterium, ↓Faecalibacterium prausnitzii, total bacteria count | 38 |
| 55 CRC patients/adjacent normal tissues | Tissue | Case-control | qPCR | ↑ETBF, Fusobacterium spp. | 39 |
| 44 CRC patients/44 healthy subjects | Tissue | Case-control | 16s rRNA | ↑Providencia, Fusobacterium | 40 |
| 97 CRC patients/48 healthy subjects | Tissue | Case-control | 16s rRNA | ↑Enterococcus faecalis, Fusobacterium spp. | 41 |
| 36 adenomas patients/18 healthy subjects | Tissue | Case-control | Quantitative real time PCR | ↑ETBF and pks(+) Escherichia coli | 42 |
| 18 CRC patients/18 healthy subjects | Tissue | Case-control | 16s rRNA | ↑Richness and diversity, Bacteroides fragilis, Parcubacteria, Bacteroides, Phascolarctobacterium, Parabacteroides, Desulfovibrio, and Odoribacter | 43 |
| 120 CRC patients/198 adenomas patients/172 healthy subjects | Fecal | Case-control | 16s rRNA | ↑Porphyromonas asaccharolytica, Peptostreptococcus stomatis, Parvimonas micra, and Fusobacterium nucleatum, ↓Lachnospiraceae | 44 |
| 31 adenomas patients/20 healthy subjects | Tissue | Case-control | 16s rRNA | ↑Lactococcus, Pseudomonas, ↓Enterococcus, Bacillus, Solibacillus | 45 |
| 511 CRC patients | Tissue | Comparative | Metagenomic sequencing | ↑Fusobacterium | 46 |
| 18 CRC patients | Tissue | Case | 16s rRNA | ↑Fusobacteria, ε-Proteobacteria | 47 |
| 65 CRC patients/adjacent normal tissues | Tissue | Case-control | 16s rRNA | ↑Fusobacteria, ↓Firmicutes, Actinobacteria | 48 |
| 52 CRC patients/47 adenomas patients/61 healthy subjects | Biopsy on mucosa | Case-control | 16s rRNA | ↑B. fragilis, Fusobacterium | 49 |
| 46 CRC patients/223 polyp subjects/231 healthy subjects | Fecal | Case-control | 16s rRNA | ↑Fusobacterium | 50 |
| 233 adenomas patients/547 healthy subjects | Fecal | Case-control | 16s rRNA | ↑Bilophila, Desulfovibrio, pro-inflammatory bacteria in the genus Mogibacterium, and multiple Bacteroidetes species | 51 |
| 11 CRC patients/12 healthy subjects | Fecal | Case-control | 16s rRNA | ↑Firmicutes, Porphyromonas, Clostridium, Ruminococcus, Selenomonas, and Fusobacterium | 52 |
| 21 adenocarcinomas patients/39 adenomas patients/14 polyps subjects/18 healthy subjects | Fecal | Case-control | 16s rRNA | ↑Actinobacteria, Firmicutes in adenomas and polyps | 53 |
| ↑Phylum Proteobacteria, family Porphyromonadaceae, Prevotellaceae, Alcaligenaceae, Enterobacteriaceae, genus Anaerostipes, Sutterella, Escherichia-Shigella in adenocarcinomas, ↓Actinobacteria, Firmicutes in adenocarcinomas | |||||
| 255 CRC patients/271 healthy subjects | Fecal | Case-control | Metagenomic sequencing | ↑B. fragilis, F. nucleatum, P. asaccharolytica, P. micra, Prevotella intermedia, Alistipes finegoldii, and Thermanaerovibrio acidaminovorans | 26 |
| 99 CRC patients/89 adenomas patients/124 healthy subjects | Fecal | Case-control | Metagenomic sequencing | ↓Bacteroides spp. and Coprococcus spp., ↑Fusobacterium, Porphyromonas, Bilophila wadsworthia | 54 |
| 89 CRC patients/16 healthy subjects | Fecal | Case-control | 16s rRNA | ↑Bacteroides, Fusobacterium, Dorea, and Porphyromonas, ↓Pseudomonas, Prevotella, Acinetobacter, and Catenibacterium | 55 |
| 99 CRC patients/96 adenomas patients/104 diverticular | Fecal | Case-control | 16s rRNA | ↓F. nucleatum and B. fragilis in adenoma | 56 |
| 25 CRC patients/25 polyps subjects/22 healthy subjects | Fecal/biopsies | Case-control | 16s rRNA | ↑F. nucleatum | 57 |
| 99 CRC patients/69 adenomas patients/77 healthy subjects | Fecal | Case-control | 16s rRNA | ↑Fusobacterium, Parvimonas, Staphylococcus, ↓Lachnospiraceae | 58 |
| 66 CRC patients/adjacent normal tissue | Tissue | Case-control | 16s rRNA | 7 microbe genera (Fusobacterium, Faecalibacterium, Akkermansia, Ruminococcus2, Parabacteroides, Streptococcus, and f_Ruminococcaceae) significantly different | 59 |
| 23 CRC patients/adjacent normal tissue | Tissue | Case-control | 16s rRNA | ↑Bacteroidetes, Firmicutes, and Fusobacterium, ↓Proteobacteria; 34 taxa differed | 60 |
| 99 CRC patients/96 adenomas patients/104 diverticular | Tissue | Case-control | 16s rRNA | ↓F. nucleatum and B. fragilis in adenoma | 56 |
| Non-bacteria dysbiosis | |||||
| 184 CRC patients/197 adenomas patients/204 healthy subjects | Fecal | Case-control | Metagenomic sequencing | ↑Basidiomycota: Ascomycota ratio, Malasseziomycetes, ↓Saccharomycetes, Pneumocystidomycetes; 38 fugal species were observed to be different | 61 |
| 74 CRC patients/29 polyps subjects/28 healthy subjects | Fecal | Case-control | 16s rRNA | ↑Ascomycota/Basidiomycota ratio, Trichosporon, Malassezia | 62 |
| 27 adenomas patients/adjacent normal tissues | Tissue | Case-control | High throughput sequencing technology | A third dominant phylum, Glomeromycota | 63 |
| 74 CRC patients/92 healthy subjects | Fecal | Case-control | 16s rRNA | ↑Diversity of the gut bacteriophage community, difference in 22 viral taxa | 64 |
| 30 CRC patients/30 adenomas patients/30 healthy subjects | Fecal | Case-control | 16s rRNA, metagenomic sequencing | Dominant by temperate bacteriophages | 65 |
| 184 CRC patients/197 adenomas patients/204 healthy subjects | Fecal | Case-control | Metagenomic sequencing | ↑Halophiles, ↓Methanogens, ↑9 archaea species | 66 |
- Abbreviations: CRC, colorectal cancer; ETBF, Enterotoxigenic Bacteroides fragilis.
2.3 Non-bacterial microbiota dysbiosis in CRC
Despite the high abundance of bacteria species in the gut microbiome, the relationship between nonbacterial microbiota dysbiosis and the progression of CRC should not be underestimated. Characterization of mycobiome, archaeome, and virome of CRC patients was also performed in the past few years. In one study, researchers characterized the gut mycobiota in 184 patients with CRC, 197 patients with adenoma, and 204 healthy subjects in Hong Kong and found that the Ascomycota/Basidiomycota ratio was decreased with the enrichment of Malasseziomycetes in CRC patients. Depletion of Saccharomycetes and Pneumocystidomycetes were also found in CRC patients compared with healthy control.61 Multi-cohort analysis of 1368 samples from 8 geographically distinct cohorts identified an increased abundance of 93 fungal species, including Candida pseudohaemulonis, Aspergillus ochraceoroseus, Aspergillus rambellii, and Malassezia globosa, whereas another 15 fungal species, including Aspergillus niger, Macrophomina phaseolina, Talaromyces islandicus, and Sistotremastrum niveocremeum, were decreased in CRC patients.67 In another study, the molecular evidence is mostly associated with inflammatory bowel disease, whereas CRC-related evidence was limited.68 Multi-cohort metagenomics suggested that A. rambellii was enriched in CRC patient and promoted tumor growth and cell proliferation.69 The abundance of Candida albicans was reported to increase in mice lacking the C-type lectin Dectin-3 upon chemical induction and may promote epithelial cell proliferation dependent on Dectin-1 and Wnt signaling.70, 71 Gut fungal commensals promote inflammasome activation and depletion of CARD9 (caspase recruitment domain 9), or kinase spleen tyrosine kinase increases the susceptibility of mice to CRC.72 Taken together, alterations in mycobiome are closely associated with CRC progression.73
Regarding the gut virome, one metagenomics analysis, including 74 CRC patients and 92 healthy controls, in Hong Kong showed that the bacteriophage diversity was increased in CRC patients; notably, 22 viral genera can be used to distinguish between patients and controls.64 Multi-cohort analysis revealed that the abundance of 115 viral species is increased, whereas 18 viral species were decreased in CRC patients.67 By constructing the random forest model to characterize the gut virome of 30 CRC patients, 30 adenoma patients, and 30 healthy controls, Hannigan et al. identified that temperate bacteriophage is dominant in CRC patients.65 In addition, by comparing primary tumor, metastatic tumor, and normal tissues in 12 cases, 5 enriched phages, including Enterobacteriaceae, Bacillus, Proteus, and Streptococcus phages, in CRC patients were identified.74 Bacteriophage can worsen colitis through TLR9 and increased IFN-γ.75 Animal studies provided evidence from another perspective. With AOM–DSS induction of CRC in mice, virome alteration was observed, and Brunovirus and Hpunavirus were positively related to tumor growth, whereas Lubbockvirus showed negative association.76
At last, the diverse archaeal community was detected in multiple human body sites and found associated with CRC as well.21, 77 Investigation of archaeome in fecal samples of CRC patients revealed the increased abundance of halophilic components, including Halorubrum, Natrinema, and Halococcus species and Euryarchaeota, associated with the depletion of methanogenic archaea, including Methanobacterium, Methanosarcina, Methanobrevibacter, Methanococcus, Methanocorpusculum, Methanococcoides, Methanosphaera, and Crenarchaeota species.66, 67 Differences in archaeal composition between mucosal samples from healthy and diseased tissues were observed in tubular adenoma and adenocarcinoma, Methanobacteriales and Methanobrevibacterium were also significantly higher in tumor tissues compared with control.78 Little is known about the role of archaea in CRC.
Collectively, the nonbacterial microbiome was demonstrated to be closely associated with CRC pathogenesis and exhibited abundance alteration in major species. However, much is still unknown about their evolution in CRC progression, and mechanistic insights are still unclear, which warrants further investigation. Noteworthy, the combination of the four kingdoms of bacteria, fungi, virus, and archaea performed better in early CRC diagnosis that provided the future clinical application of microbiota in CRC, which highlighted the critical role of non-bacteria in CRC.67
2.4 Interaction between multi-kingdom microbiota
In addition to single-kingdom microbiota analysis, interactions across multiple-kingdom microbiota can also provide valuable insights into CRC. Compared to healthy controls, higher numbers of co-occurring fungal intra-kingdom and co-exclusive bacterial–fungal correlations were found in CRC patients revealed by ecological analysis. Moreover, co-occurrences between fungi and bacteria, mostly contributed by fungal Ascomycota and bacterial Proteobacteria in control, were reverted to co-exclusive interplay in CRC.61 Besides, interactions between bacteriophages and oral bacterial commensals in patients with CRC were changed compared with controls, and the survival rate of Streptococcus species was regulated by the bacteriophage community.64 These results provide fundamental evidence that bacteriophage communities are associated with CRC and potentially impact cancer progression by altering the bacterial communities.65 Moreover, archaea and bacteria exhibit significant ecologic relationships in CRC patients as well. CRC-associated halophiles were positively related to the oncogenic bacterium B. fragilis and were negatively associated with butyrate-producing Clostridium species.66 Monozygotic and dizygotic twin pairs study reported that methanogenic archaea were positively correlated with bacteria and their mutualism was supported by animal studies.79, 80 Although extensive research is still required, current studies suggest that inter-kingdom interactions among archaea, fungi, viruses, and bacteria contribute to colon tumorigenesis and are worthwhile to be investigated.
2.5 Aging-related microbiota in CRC
The vital effect of microbiota in the progression of aging has not yet been completely investigated, and Paul et al. provided evidence that the reduction of core microbiota group diversity is related with increased frailty but not significantly associated with chronological aging (Table 2).81 Several cohort studies also support the revealed depletion in core enrichment taxa (Bacteroides, Roseburia, and Faecalibacterium spp., among others) while rare taxa increased.15, 82, 83 In a cohort study compromised of centenarians, older people showed high levels of Firmicutes population and facultative anaerobes. Intriguingly, increased oral microbiota abundance, Bifidobacterium species, and Eubacterium limosum, which were considered beneficial microbiota, were also reported as microbial markers of aging in recent cross-cohort studies.84, 87 In addition to the altered diversity, increased α diversity was also reported reflecting the complex and dynamic alteration of microbiome with aging.85, 86 Given the altered microbial species pattern in aging is not consistent with that in CRC, more studies are needed to investigate the relationship among microbiota, aging, and CRC.
| Microbiota dysbiosis | |||||
|---|---|---|---|---|---|
| Sample size | Sample type | Study design | Microbiota analysis | Main finds comparing cases to controls | References |
| 30 centenarians, 17 elderly and 9 adults | Fecal | Comparative | 16s rRNA | ↑Firmicutes, Escherichia, Akkermansia, Clostridium, and Collinsella, ↓Faecalibacterium and Prevotella | 82 |
| 21 centenarians, 25 elderly and 19 adults | Fecal | Cross sectional | Metagenomic sequencing | ↑Pyramidobacter, Desulfovibrio and Methanobrevibacter, ↓Faecalibacterium, Ruminococcus, Coprococcus, and Dorea | 83 |
| 751 older than 50-year old, 990 smaller than 55-year old | Fecal | Cross-sectional | Metagenomic sequencing | ↑Oral Streptococci, Lactobacillus salivarius, Actinomyces viscosus, Solobacterium moorei, Clostridium perfringens; Campylobacter concisus, Veillonella atypica, Veillonella parvula, Klebsiella, Citrobacter, Firmicutes, and Bifidobacterium | 84 |
| 67 centenarians, 54 elderly and 47 adults | Fecal | Cross-sectional | 16s rRNA | ↑Clostridium cluster XIVa, Ruminococcaceae, Akkermansia, and Christensenellaceae | 85, 86 |
3 DIET AND MICROBIOTA MODULATION
Diet plays a vital role in regulating gut microbiota; specifically, dietary preferences are closely associated with the composition of microbiota in CRC patients.16, 88 A meta-analysis study showed that a healthy diet, including fruits and vegetables, whole grains, nuts and legumes, fish and other seafood, milk, and other dairy products was associated with a low risk of CRC incidence.89 In contrast, an unhealthy diet characterized by a high intake of red meat, processed meat, sugar-sweetened beverages, refined grains, desserts, and potatoes was associated with a higher risk of CRC.90 Alaska natives whose diets were characterized by high fat, high animal protein, and low fiber in their diet have the highest risk of sporadic CRC91; analysis of their fecal samples showed the compositional predominance of Blautia and Lachnoclostridium, which have high activity in bile acid (BA) conversion, whereas the low abundance of species involved in saccharolytic fermentation like Prevotellaceae and Ruminococcaceae.91 By comparing the bacteria composition and dietary mode between African–American (AA) and non-Hispanic Whites (NHWs) CRC cases, it showed that the AA population had higher fat and protein intake and enrichment of sulfidogenic bacteria like Bilophila wadsworthia and Pyramidobacter spp. in their gut than NHWs, which are associated with higher CRC incidence.92 To explore the role of different dietary interventions and probiotics in CRC development, several studies were performed, and their discoveries are summarized here.
3.1 Mediterranean diet
Mediterranean-style diet (MED) is nutritionally balanced, characterized by large amounts of monounsaturated fatty acids (olive oil); fibers (cereals, vegetables, legumes, and nuts) and fruit; moderate consumption of seafood and red wine.93 Case-control studies reported MED adherence is associated with decreased CRC risk94, 95; however, the results of cohort studies are inconclusive.96-98 Study design, age, ethnicity, and gender of the population may contribute to controversial observations.99 However, meta-analysis supported the overall protective effect of MED on CRC.93, 100 Variable nutrients in MED positively modulate the intestinal microbial composition, which is beneficial in preventing CRC. Specifically, it helps promote microbial diversity and secretion of anti-inflammatory metabolites like short-chain fatty acids (SCFAs).101-104 MED-associated bacterial taxa were enriched and occupied keystone interaction positions in the microbiome ecosystem network in the gut after a 12-month MED intervention.105 Higher levels of Actinobacteria, as well as lower levels of Proteobacteria, Fusobacteria, and Firmicutes–Bacteroidetes ratios, were observed after MED administration.106-108 Depletion of metabolic endotoxemia and maintenance of gut barrier integrity is, on the other side, another beneficial effect of MED in improving metabolic health.109, 110 Anti-inflammatory and immune-modulating activities of MED are possible molecular mechanisms for reducing the risk of CRC.111, 112 Typical food components in MED as well as their anti-CRC effects will be discussed subsequently.
3.1.1 Fiber
Dietary fiber is defined as the component of vegetarian food that is hard to be digested by human body, including polysaccharides, inulin, resistant starches (RSs), and oligosaccharides.113 Numerous studies have established the connection between high fiber intake with the low risk of CRC.114-116 One hypothesis is that fiber exerted a positive effect through the modulation of gut microbiota and upregulation of butyrate production. Butyrate was reported to inhibit the activity of histone deacetylases and associated signaling pathways, which promote cancer cell apoptosis by imposing synergistic inhibitory and anti-inflammatory effects.117 This hypothesis was further supported by Chen et al. who also found that high-fiber dietary patterns along with consistent production of SCFAs by healthy gut microbiota were associated with a reduced risk of advanced colorectal adenoma.118 Another clinical study found that higher fiber intake tended to be associated with the accumulation of genera of Clostridia, including the higher abundance of SMB53, Lachnospira, and Faecalibacterium, whereas with the decreased amount of Actinomyces, Odoribacter, and Oscillospira.119 However, a meta-analysis of 64 studies involving 2099 participants demonstrated the effect of dietary fiber intervention in the enrichment of Bifidobacterium spp. and Lactobacillus spp., without interfering with SCFA secretion.120
Different kinds of fiber have different effects on gut microbiota. Inulin has been shown to increase the abundance of probiotics, including Lactobacillus spp. and Bifidobacteria, and reduce the biomass of pathogenic bacteria like Escherichia coli and Salmonella enterica serovar Typhi in mice.121 Mice fed with inulin had approximately 50% colonic tumors compared with mice fed with cellulose, and the abundance of Bacteroides genus was reversely correlated with tumor burden.122 RS and its digestive products escaping from the small intestine also contribute to the modulation of gut microbiota.123 Although there was no direct evidence from human studies, numerous animal studies highlighted the effect of RS in preventing CRC development. RS administration raises the level of a range of bacteria that is important for starch degradation and SCFA production.124 RS feeding shifts microbial composition, characterized by the increase of Parabacteroides, Barnesiella, Ruminococcus, Marvinbryantia, and Bifidobacterium, which promotes SCFA production and expression of SCFA receptor GPR43.125, 126 RS was also found beneficial in alleviating carcinogenic effects of the western diet by increasing the level of SCFA and reducing the level of ammonia and phenol.127 It was also reported that butylated starch intake can prevent red meat–induced O6-methyl-2-deoxyguanosine adducts in human rectal tissue.128 In addition, prudent diets rich in whole grains and dietary fiber are associated with a lower risk of F. nucleatum-positive CRC but not F nucleatum-negative cancer.129 Pectin, a widely consumed soluble fiber, helps enrich butyrate-producing bacteria that promotes anti-PD-1 mAb efficacy in CRC treatment through regulatory T cell infiltration.130 Taken together, considering the diverse sources of fiber and different functions, further studies are needed to comprehensively investigate the mechanism of fiber-induced microbiota modulation and their role in CRC.
3.1.2 Dietary fatty acids
Fatty acids are another important source of nutrients in daily diet, and their roles in gut microbiota modulation and CRC progression have been investigated. Recent evidence supports the potential benefit of marine-3 fatty acids in preventing CRC.131, 132 A randomized trial of 8-week treatment with 4 g mixed omega-3 polyunsaturated fatty acids separated by a 12-week washout period in mice showed a reversible increase in SCFA-producing bacteria, including Bifidobacterium, Roseburia, and Lactobacillus, which has anti-CRC activity.133 Except for that, an observational study of middle-aged to older women revealed that serum omega-3 levels are related to intestinal microbiome diversity and the abundance of specific bacteria, especially for butyrate-producing Lachnospiraceae family.134 EPA–FFA treatment restored the loss of the Notch signaling pathway in the AOM–DSS-induced CRC model and enriched the amount of Lactobacillus species in gut microbiota.135 Another important source of fatty acids is olive oil. Intake of olive oil polyphenols favors a healthy gut microbiota, characterized by the increase of Bifidobacteria and intestinal IgA-coated bacteria.136 When comparing olive oil with coconut and sunflower as the only source of fatty acids, the olive oil–containing diet produced an anti-inflammatory microenvironment characterized by a reduced level of Enterococcus, Staphylococcus, Neisseria, and Pseudomonas spp., and an increase of Firmicutes/Bacteroidetes ratio.137 The higher plasmatic concentration of oleic, α-linolenic, and linoleic acids exhibits a significant association with lower CRC risk according to the Singapore Chinese Health Study.138 Taken together, unsaturated fatty acids are beneficial in preventing CRC through microbiota regulation.
3.1.3 Fruit
Although there is no direct evidence regarding the role of fruit in CRC, supplementation with different kinds of fruit was proved to be associated with certain changes in intestinal microbiota. Colorful vegetables and fruits containing anthocyanins reduced the abundance of pro-inflammatory B. wadsworthia, which lead to the attenuation of microbiota dysbiosis in CRC mice.139, 140 Berries are rich in phenolic compounds, such as phenolic acids, flavonols, and anthocyanins, which were demonstrated to modulate microbial population, characterized by the increase of Bifidobacterium, Lactobacillus, and Akkermansia.141 Anti-inflammatory bacteria, such as Akkermansia, Desulfovibrio, and butyrate-producing bacteria–like Anaerostipes, were increased following whole freeze-dried black raspberry administration.142 A daily dose of 200–400 g of mango pulp for 8 weeks significantly increases the amount of Lactobacillus spp., Lactobacillus plantarum, L. reuteri, and Lactobacillus lactis, which have been shown to participate in butyric acid production.143 The animal-based diet increased the abundance of bile-tolerant microorganisms (Alistipes, Bilophila, and Bacteroides) and reduced the levels of Firmicutes that metabolize dietary plant polysaccharides (Roseburia, Eubacterium rectale, and Ruminococcus bromii). Nevertheless, additional studies are required to identify the critical compounds of fruit to optimize the protective effects against CRC.
Taken together, the MED diet can increase the abundance of probiotics, including Bifidobacterium spp. and Lactobacillus, which were decreased in CRC. That would be the possible protective mechanism of MED diet in CRC. Besides, MED diet can promote the production of metabolites like SCFA that holds great promise in protecting against CRC as the decreased amount was observed during CRC progression.144
3.2 Nordic diet
Nordic diet (ND) is another dietary habit that favors traditional, locally grown, and seasonal foods of the Nordic countries, characterized by the intake of whole grains, including oats, rye, barley, and specific fruit and vegetables, including apples/pears, berries, root vegetables, cabbages, and fish.145, 146 MED and ND are similar, except for the difference in oil used from each other. Olive oil is widely used in MED, whereas rapeseed oil is commonly seen in ND, leading to a higher level of monounsaturated fatty acids in MED.147, 148 Longitudinal epidemiological studies focusing on the role of ND in preventing chronic disease are limited and few conclusions have been drawn by far. One Danish cohort study showed that a higher level of ND consumption was related to a lower risk of CRC in women rather than in men, whereas the result from another Swedish cohort was against it, which showed no association in women.149, 150
3.3 Western diet
In contrast to MED, the western diet is characterized by a high intake of red or processed meat, refined grains, and sugar-sweetened beverages, whereas a low consumption of fresh fruits, vegetables, and legumes.151 Red meat has been demonstrated to increase the risk of CRC.152 N-Glycolylneuraminic acid (Neu5Gc) and heme are specifically enriched in red meat, and the overconsumption of red meat leads to the accumulation of Neu5Gc that disrupts the homeostasis of gut microbiota characterized by the upregulation of Bacteroidales and Clostridiales.153 Mice fed with a heme-supplemented diet lead to a significant alteration of gut microbiota, characterized by the decrease in α-diversity, reduction of Firmicutes, increase of Proteobacteria, particularly Enterobacteriaceae, and reduction in fecal butyrate levels.154 In human studies, red meat diet has been shown to increase fecal Bacteroides, which increases the risk of CRC together with Streptococcus bovis, Fusobacterium, Clostridium, and Helicobacter pylori; meanwhile, L. plantarum, Lactobacillus acidophilus, and Bifidobacterium longum that can restrain the process were reduced.155 Another characteristic of the western diet is high fat. The high-fat diet (HFD) promotes the hepatic synthesis of BAs and increases its delivery efficiency to the colonic lumen, which stimulates the growth and activity of 7α-dehydroxylation bacteria, and promotes the conversion of primary BAs into secondary BAs, especially deoxycholic acid (DCA).156 The influence of diet on BA conjugation varies. For instance, vegetarian diets favor glycine conjugation, whereas diets high in animal protein favor taurine conjugation. Digestion of taurine-conjugated BAs by gut microbes generates hydrogen sulfide, which was shown to promote CRC progression as a genotoxic compound.156 Compared with NHWs, AAs have a significantly higher abundance of sulfidogenic bacteria and B. wadsworthia-specific dsrA along with higher fat and protein intake and daily servings of meat of AAs.157 By using a sulfur microbial dietary score to investigate the relationship between diet mode and CRC incidence, it was found that long-term adherence to a particular dietary mode favoring sulfur-metabolizing bacteria in stool was associated with an increased risk of distal CRC,158 which was further supported by animal studies comparing the microbiota composition of rats feeding with a normal diet or HFD, which showed an altered microbiota composition in HFD group characterized by the reduction in Ruminococcus, Candida, Saccharomyces, Enterobacter, Clostridium IV, Enterococcus, Enterobacter, Vibrioaceticus, and so on, which leads to an inflammatory condition of host.159 High salt intake is another feature of western diet, whereas high salt diet was reported to inhibit colonic inflammation, in-line with the reduction of enterotoxigenic B. fragilis (ETBF)-mediated tumorigenesis through decreasing the expression of IL-17A and inducible nitric oxide synthase.160 Taken together, the western diet–induced microbiota pattern is close to the one under CRC-associated microbiota, with the increase of Fusobacterium, Bacteroid, and ETBF and decrease of Bifidobacterium and Lactobacillus. However, the impact of diets on gut bacteria and CRC is complicated, and more studies are required to clarify their relationships.
3.4 Interaction between nonbacterial microbiome and diet
In contrast to gut bacteria, few studies have shown the effect of diet on the nonbacterial microbiome.161 The field of nonbacterial microbiome lags gut bacterial research, and most of the data were collected from healthy participants rather than CRC patients. According to a study of 98 healthy adults, the consumption of carbohydrate-rich food was correlated with an increase in Candida spp. and Methanobrevibacter, whereas diets high in amino acids, protein, and fatty acids had opposite effects.162 Analysis of healthy participants with a vegetarian diet showed that Fusarium was the most abundant genus, followed by Malassezia, Penicillium, Aspergillus, and Candida.163 The HFD was correlated with a reduced abundance of Alternaria, Saccharomyces, Septoriella, Tilletiopsis genera, Saccharomyces cerevisiae, and Tilletiopsis washingtonensis in mice.164 Halophiles are enriched in salty fish and seafood.165 Research revealed that the intake of salty foods promotes the enrichment of halophilic archaea in the gut, which is associated with a higher rate of colon cancer incidence.166 The abundance of Methanobrevibacter was higher in healthy people, associated with the high intake of fiber, whereas it was relatively low in patients with rheumatoid arthritis and was reversed by MED diet consumption.102, 167 For virome, dietary interventions consistently affect the composition and proportions of viral species in the gut.168 HFD leads to an increase of temperate phages under Caudovirales order.169, 170 These results demonstrated that fungi, archaea, and viruses are affected in response to diet intervention. However, further studies are still required to understand the mechanisms of different diets in modulating nonbacterial microbes.
4 PROBIOTICS
Probiotics are defined as, “live microorganisms that, when administered in adequate amounts, confer a health benefit on the host.”171 Most probiotics belong to lactic acid bacteria and yeast populations.172 Bifidobacterium metabolites were shown to inhibit the growth of cancer cells.173 The anticarcinogenic activity of probiotics is associated with the modification of intestinal microbiota composition, production of beneficial metabolites, such as SCFAs and conjugated linoleic acids, and immunomodulation activity in reducing inflammation.174 Meta-analysis showed that the administration of probiotics altered the microbiota composition, with the enriched abundance of beneficial bacteria (Lactobacillus, Eubacterium, Peptostreptococcus, Bacillus, and Bifidobacterium), and reduced the level of potentially harmful bacteria (Fusobacterium, Porphyromonas, Pseudomonas, and Enterococcus).175 Genaro et al. also revealed that the supplementation of the probiotic mix can attenuate the aggressiveness of cancer.176 The correlation of a high Enterococci abundance with a low risk of CRC development suggests that certain Enterococcus strains may have protective effects against adenoma.177 L. reuteri, another potential probiotic adjunct, was shown to protect against CRC as well.34
Probiotics modulate gut microbial community and promote the production of beneficial metabolites. Studies showed that the intake of yogurt containing B. longum (BB536-y) and fructooligosaccharides increased the total amount of SCFA in the feces and significantly suppressed the amount of ETBF as well as the growth of putrefactive bacteria.178 Animal studies showed that Lactobacillus suppressed the enzyme activities of β-glucuronidase and β-glucosidase, reduced the amount of Ruminococcus sp., Clostridiales bacteria, E. coli, Bacteroides dorei, and increased the amount of Prevotella that promoted the reversion of the gut microbiota close to healthy state.179, 180 C. butyricum inhibited intestinal tumor development by modulating Wnt signaling in addition to gut microbiota, demonstrated by the depletion of some pathogenic bacteria and BA bio-transforming bacteria, and the accumulation of beneficial bacteria, including SCFA-producing species.36 Administration of Bifidobacterium improved the efficiency of anti-CD47 immunotherapy and converted nonresponder mice into responders to stimulators of interferon genes.181 Intake of yeast probiotics enhanced the therapeutic efficiency to CRC with reduced adverse effects that had been reviewed elsewhere.182 Regarding the yeast, S. cerevisiae and Saccharomyces boulardii are common species found in CRC. Studies showed that S. cerevisiae induced apoptosis and inhibited tumor growth or metastasis,172, 183 whereas S. boulardii (Sb) exhibited an anti-inflammatory effect by inhibiting the production of IL-8 and NF-kB activity in HT-29 cells.184 Collectively, the intake of probiotics induces the alteration of microbiota to protect CRC, whereas the effectiveness and safety of probiotics need to be addressed by additional research.
5 MICROBIOTA AND CRC THERAPIES
5.1 Radiotherapy
Radiotherapy is one of the most frequently used treatments in CRC, and microbiota plays important role in CRC radiotherapy.185, 186 An animal study revealed the increased abundance of commensal Alistipes along with the decreased amount of Mucispirillum genus in mouse gut after radiation.187 β-diversity significantly differed between complete response (CR) and non-CR patients. Specifically, a higher abundance of Bacteroidales (Bacteroidaceae, Rikenellaceae, and Bacteroides) was observed in non-CR than CR patients after concurrent chemoradiation in rectal cancer. Duodenibacillus massiliensis was associated with an improved CR rate.188 It showed that GF mice are resistant to lethal radiation that suggests the role of microbiota in regulating intestinal radiosensitivity.189 Meta-analysis revealed notable changes in intestinal microbiota characterized by the decrease in Bifidobacterium, Clostridium cluster XIVa, and Faecalibacterium prausnitzii and an increase in Enterobacteriaceae and Bacteroides after radiotherapy.190 Taken all, altered bacterial composition after radiotherapy has implied the potential effects of bacteria in radiotherapy while the exact mechanism is still under-explored. Other components of intestinal microbiota are also related to radiotherapy effectiveness. Polyphenol has been discovered as a natural radioprotective product.191 In addition to gut bacteria, distal bacteria can also translocate to CRC sites and affect the efficiency of treatment, as it showed that F. nucleatum can migrate to CRC tissue and impairs radiotherapy efficacy.192
Commensal fungi also regulate the responsiveness of tumor cells to radiation therapy for lung and breast cancers through the modulation of T cell activity. Depletion of commensal fungi enhances the efficacy of radiation therapy, whereas commensal bacteria had exactly the opposite effect.193 Mice orally administrated with S. cerevisiae-derived-beta-d-glucan before irradiation showed decreased ROS level, mitigated DNA damage, and apoptosis, which was associated with an increased concentration of radioprotective cytokines in plasma. Supplementation of S. cerevisiae derived-beta-d-glucan had radioprotective effects, and the side effects were reduced accordingly.194 Overall, the specific mechanism of fungi species in regulating radiotherapy efficiency needs to be addressed.
5.2 Chemotherapy
Platinum derivatives (oxaliplatin), antimetabolites (5-fluorouracil; 5-FU), cyclophosphamide (CTX), and topoisomerase inhibitors (irinotecan) are the most used agents in CRC chemotherapy, which also shown effects on microbiome.195 Disruption of microbiota through antibiotics reduced the effectiveness of oxaliplatin and cyclophosphamide in MC38 tumor-bearing mice.196 However, the therapeutic efficacy was affected by F. nucleatum through autophagy, which contributes to resistance to oxyplanin and 5-FU.197 Conversely, 5-FU also affected microbiome composition characterized by the depletion of Eubacterium and Ruminococcus. Viaud et al. showed that CTX administration induced the translocation of Gram-positive bacteria (mainly Lactobacillus johnsonii and Enterococcus hirae) from the small intestine to secondary lymphoid organs enriched with effector pathogenic Th17 cells compared with control.198 Consistently, Daillère et al. also showed E. hirae translocation associated with increased intratumoral CD8/Treg ratio during CTX therapy. Barnesiella intestinihominis is also involved in anticancer immune responses and promoted the infiltration of IFN-γ-producing γδT cells.199 Intratumor bacteria are also important in CRC therapy. Γ-Proteobacteria within tumor metabolized gemcitabine into an inactive form, which results in drug resistance, whereas it can be reversed by co-treatment with antibiotic ciprofloxacin.200 Besides, β-glucuronidase produced by intestinal bacteria disrupted irinotecan metabolism by turning inactive SN-38G into the active form of SN-38 and causing intestinal damage.201 Microbiota can be used as a biomarker to predict the efficiency of chemotherapy as it exhibits distinct profiles between nonresponders and responders. According to a study of patients receiving neoadjuvant chemoradiotherapy, Shuttleworthia was found enriched in responders, whereas several other bacteria taxa under the order of Clostridiales and so on were enriched in nonresponders.202 In-line with this, certain fungi species are also important in regulating chemotherapy efficacy. For instance, Candida tropicalis increased the resistance of colon cancer to oxaliplatin by producing lactate and inhibiting the expression of mismatch repair system effectors.203
5.3 Immunotherapy
Immunotherapy is approved for the treatment of CRC patients with high microsatellite instability.204 Prevotella and Akkermansia are beneficial in maintaining the efficacy of PD-1 treatment by regulating the metabolism of glycerophospholipid after antibiotic treatment in CRC tumor-bearing mice.205 Likewise, the alteration of microbiota promoted IL-1β and IL-6 signaling pathways to induce protective intestinal Th17 cells in Tak1-deficient (transforming growth factor-β-activated kinase-1, Tak1ΔM/ΔM) mice. Besides, Odoribacter splanchnicus, which was enriched in Tak1ΔM/ΔM mice, exhibited protective effects on wild-type mice by inducing Th17 cells.206 A total of 11 bacteria strains isolated from healthy human feces induced interferon-γ-producing CD8 T cells and enhanced immune checkpoint inhibitors (ICI) effectiveness in mice colon cancer models.207 Although The associations between anti-inflammatory bacterial metabolites SCFA and the efficiency of ICI remain unclear,186, 208 studies have shown that higher fecal and plasma levels of SCFA were related to longer progression-free survival (PFS) in patients with solid tumor who were treated with anti-PD-1 antibody.209 Another metabolite associated with ICI efficiency is inosine, which is produced by Bifidobacterium pseudolongum and is proven to enhance immunotherapy efficiency in animal models.210 Total membrane fractions and peptidoglycans (PDGs) in Porphyromonas gingivalis induced the upregulation of PD-L1 expression in colon cancer cells.211 On the other side, Bacteroides thetaiotaomicron and B. fragilis were associated with the efficacy of CTLA-4 blockade therapy. Oral gavaging with B. fragilis, immunization with B. fragilis polysaccharides, or adoptive transfer of B. fragilis–specific T cells efficiently resensitized antibiotic-treated or germ-free mice in response to anti-CTLA treatment.212 However, anti-CTLA-4 antibody treatment cohorts in French and Italian show propionate concentrations were correlated with shorter PFS.213
Prophage is also associated with the efficiency of immune therapy. Fluckiger et al. reported that the administration of Enterococci harbor bacteriophage enhanced T cell responses after PD-1 blockade treatment in a mouse model. To expand the discovery to humans, they also compared the metagenomics data of advanced renal and lung cancer patients with healthy controls and revealed that detectable fecal prophage was related to a prolonged overall survival rate after anti-PD-1 therapy.214 As above, differences in the composition or activity of nonbacterial microbes may contribute to different responsiveness in immunotherapies against CRC, although more in-depth studies are still needed.
6 FUTURE PERSPECTIVE
Here, we reviewed recent discoveries focusing on human microbiota and CRC (Figure 1). By focusing on the research of human gut microbiota, we are trying to provide a comprehensive view on the role of multi-kingdom microbiota dysbiosis on CRC progression. Accumulating evidence highlights the relationship between microbiota and the efficiency of CRC therapies, whereas few studies investigated the role of nonbacterial species, including fungi, viruses, and archaea. Diet intervention is the one of most convenient solutions in CRC prevention and treatment through gut microbiota modulation. A healthy diet mode like MED, which is characterized by high consumption of fiber and unsaturated fatty acid, could be a promising way to improve colonic health and prevent carcinogenesis. Fecal microbiota transplant is another potential way to improve the outcomes of CRC patients and reduce the risk of toxicity or any other side effects induced by current treatments. Chang et al. revealed that fecal microbiota transplantation (FMT) significantly reduced the severity of diarrhea and intestinal injury, which are commonly seen in 5-FU-based chemotherapy.215 Fecal donations from healthy volunteers containing the mixture of bacteria, fungi, viruses, and archaea may have stronger benefits than single-strain administration. However, the selection of volunteers, safety, and compatibilities between donors and recipients remain elusive, which limits the application of FMT technologies. Antibiotic, another microbiota-manipulating strategy, is frequently used in cancer treatment. Some studies have shown different outcomes of cancer patients with or without antibiotics administration.186 Notably, the impact of nonbacterial microbiota dysbiosis caused by antibiotics on therapy efficacy still needs to be addressed. To expand our knowledge on the role of microbiota in CRC progression and treatment, future studies are needed to comprehensively characterize the composition and activities of microorganisms (bacteria, fungi, and viruses) under various conditions. A deep understanding of multi-kingdom microbiota interactions will pave the way to accurately manipulate microbiota in treating CRC patients.
AUTHOR CONTRIBUTIONS
Data curation (lead); writing—original draft (lead): Yue-Mei Hong. Writing—review and editing (supporting): Dingka Song. Conceptualization (equal); supervision (equal); writing—review and editing (equal): Ning-Ning Liu and Hui Wang.
ACKNOWLEDGMENTS
This study was supported by research funding from the National Natural Science Foundation of China, Grant/Award Number: 31900129, 82030099, 81630086, and 32200749; National Key R&D Program of China, Grant/Award Number: 2018YFC2000700; Shanghai Public Health System Construction Three-Year Action Plan, Grant/Award Number: GWV-10.1-XK15; Innovative Research Team of High-level Local Universities in Shanghai.
CONFLICT OF INTEREST STATEMENT
The authors declared no potential conflict of interests concerning the research, authorship, and/or publication of this article.
ETHICS STATEMENT
The authors declared that ethics is not applicable as no experiments involved in the study.
Open Research
DATA AVAILABILITY STATEMENT
Data sharing is not applicable to this article as no new data were generated in this study.




