Hybrid nanosystems for the treatment of infectious diseases produced by multidrug-resistant bacteria: A systematic review
Abstract
The rise of multidrug-resistant (MDR) bacteria poses a significant challenge to effectively treating infectious diseases. In recent years, nanotechnology has emerged as a promising approach to combat MDR bacterial infections. This systematic review, following the research guidelines proposed by PRISMA, aims to provide a comprehensive overview of the research conducted on hybrid nanosystems for the treatment of infectious diseases caused by MDR bacteria. Various categories of hybrid nanosystems combining different nanomaterials were investigated, including gold-, carbon-, and silver-based materials; iron oxide; copper oxide nanoparticles; liposomes; and polymers. Each category was thoroughly examined, and the studies were analyzed based on their methodologies, findings, and potential applications. The review highlights the progress made in the development of hybrid nanosystems, their unique properties, and their effectiveness in combating MDR bacterial infections. The findings from this systematic review provide valuable insights into the field of infectious disease management, aiding in the identification of potential nanosystem candidates for further development and clinical translation.
1 INTRODUCTION
Multidrug-resistant (MDR) bacteria are a major public health concern due to their ability to withstand multiple types of antimicrobial agents.1 This resistance is often acquired due to genetic mutations or acquired through genes from other bacteria and can occur as a result of the overuse or misuse of antibiotics.
The problem of MDR bacteria has become increasingly urgent in recent years, as the number of antibiotic-resistant infections continues to increase. According to the World Health Organization (WHO), antibiotic resistance is one of the biggest threats to global health, food security, and development today.2 The emergence of new and more virulent strains of MDR bacteria, such as carbapenem-resistant Enterobacteriaceae (CRE)3 and methicillin-resistant Staphylococcus aureus (MRSA),4 underscores the need for new approaches to combat these infections.
Hybrid nanosystems,5 also known as nanohybrids or nanocomposites, are a class of systems that combine two or more different types of nanomaterials into a single, integrated structure. These nanosystems can include different nanomaterials such as quantum dots, liposomes, and polymers, among others.
Hybrid nanosystems offer a promising approach to address the problem of MDR bacteria, as they can be designed to target specific bacterial strains and deliver antimicrobial agents or improve the immune response.6 By improving the specificity and efficacy of antimicrobial treatments, they have the potential to reduce the incidence and severity of MDR bacterial infections and improve patient outcomes.
- Their small size and high surface area-to-volume ratio make them ideal for delivering antimicrobial agents directly to bacterial cells. This can increase the efficacy of the treatment while minimizing the risk of side effects or damage to healthy cells.
- They can be designed to specifically target MDR bacteria by incorporating ligands or other targeting moieties that bind to specific bacterial cell surface markers. This can enhance the selectivity and specificity of the treatment, while minimizing the risk of resistance development.
- They can be customized to enhance the immune response to MDR bacteria, by incorporating immunostimulatory agents or adjuvants, which activate the immune system.8 This can help to overcome the immune evasion strategies used by some MDR bacteria and improve the effectiveness of antimicrobial treatments.
- They can be tailored to overcome the physical and chemical barriers that often limit the efficacy of traditional antimicrobial agents, such as poor solubility or rapid clearance from the body. By improving the pharmacokinetic and pharmacodynamic properties of antimicrobial agents, hybrid nanosystems can increase their effectiveness against MDR bacteria.
Furthermore, this therapy involves the simultaneous or sequential use of multiple therapeutic agents or strategies within a single hybrid nanosystem. The synergy obtained by combining different therapeutic agents, such as drugs, nanoparticles (NPs), peptides, or enzymes, in a single system allows for enhanced antimicrobial activity.
Moreover, these systems can serve as effective carriers for targeted and controlled drug delivery and can function as theranostic platforms that integrate therapeutic and diagnostic functions. They adapt to individual patient characteristics, allowing for personalized medicine.
In summary, the combination therapy approach using hybrid nanosystems holds great promise in the field of infectious diseases. It offers opportunities to improve treatment outcomes, overcome drug resistance, and provide personalized and targeted therapies. Ongoing research and development in this area aim to further optimize the use of hybrid nanosystems for the effective and precise treatment of infectious diseases.
- Evaluate hybrid nanosystems designed to treat infections caused by drug-resistant bacteria, analyzing their characteristics.
- Assess the effectiveness of nanosystems against MDR bacteria, examining mechanisms, efficacy, safety profiles, testing methods, identification, limitations, optimization, and in vivo/in vitro models.
- Identify gaps or limitations in current research on these nanosystems for drug-resistant infections, aiming to guide future improvements and advancements.
2 METHODS
This systematic review followed the PRISMA guidelines to ensure methodological rigor and transparency. A comprehensive search of PubMed, ScienceDirect, and Scopus databases was conducted to identify studies on hybrid nanosystems targeting infectious diseases caused by MDR bacteria, focusing on publications from 2013 to 2023. Figure 1 illustrates the PRISMA flowchart used for the article selection process.

Inclusion criteria encompassed studies involving hybrid nanosystems specifically designed to target MDR bacteria, reporting outcomes such as efficacy, safety, mechanisms of action, and characterization. Both in vitro and in vivo studies were considered, along with clinical studies where available. Exclusion criteria eliminated review articles, conference abstracts, opinion pieces, and studies unrelated to MDR bacterial infections or published in languages other than English.
A two-step screening process was applied: titles and abstracts were initially reviewed for relevance, followed by a full-text evaluation of potentially eligible studies. Discrepancies were resolved through consensus.
3 RESULTS AND DISCUSSION
3.1 Results and hybrid nanosystems classification
A total of 40 results were recovered from the Science Direct database. From the PubMed database, 35 results were obtained. Additionally, the Scopus database yielded 50 results. No articles from other sources were included in the analysis. Following the exclusion process, a total of 83 articles were chosen. Among these records, 62 met the eligibility criteria for this study.
3.2 Synthesis and characterization of nanohybrid systems
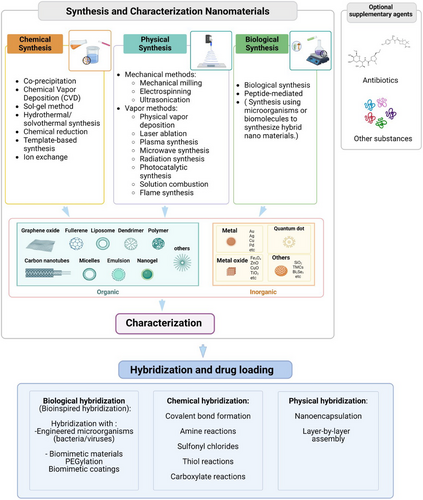
In this systematic review, we illustrate the meticulous process of preparing and evaluating hybrid nanoantibacterial agents, emphasizing their progression toward clinical trials. Our depiction of this journey is presented through four key figures, each crafted to highlight crucial aspects of the developmental pathway. Figure 2 focuses on the foundational stages of preparing hybrid nanoantibacterial agents. This figure serves as a roadmap for the synthesis of nanoantibacterial agents, outlining the various synthesis methods used to create NPs, which are the fundamental building blocks for these agents. The synthesis methods encompass a wide range of techniques, including chemical, physical, biological, and layering methods, each leading to the formation of different kinds of organic and inorganic NPs. This figure emphasizes the importance of characterizing these NPs before proceeding with the hybridization step. After hybridization, where supplementary agents are integrated using diverse methods such as biological, chemical, and physical techniques, it is crucial to re-evaluate and characterize the materials. This posthybridization characterization ensures a comprehensive understanding of the modified nanoantibacterial agents and their enhanced properties.
The characterization of these nanoantibacterial agents is of paramount importance to unravel their intricate structural, chemical, and physical properties. This comprehensive characterization utilizes an array of specialized devices. As described in Figure 3, each of these techniques contributes to different facets of characterization.
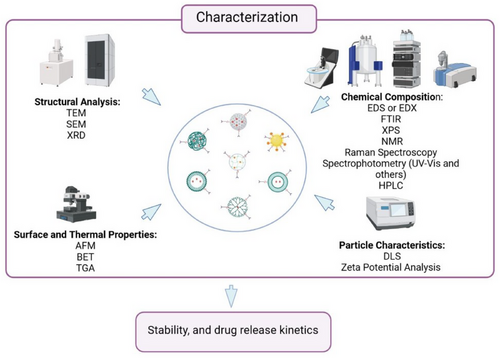
The comprehensive array of characterization techniques includes structural analysis, employed to precisely discern the size, shape, and overall structure, which is crucial to identifying specific characteristics that contribute to antibacterial effectiveness. Chemical composition analysis techniques reveal the chemical bond and elemental makeup, confirm their composition, and detect functional groups that may influence antibacterial properties. Measurements of particle size and dispersion reveal the uniform distribution within the intended medium, a key factor ensuring their effectiveness. Surface properties analysis provides valuable insights into topography, roughness, and mechanical properties, shedding light on interactions with bacterial cell surfaces.
Thermal behavior insights into stability, decomposition patterns, and potential weight loss under varying thermal conditions, coupled with optical properties analysis, which examines how agents interact with light under various conditions—both collectively offer a nuanced understanding of these agents’ properties.
Following thorough characterization of nanoantibacterial agents, subsequent pivotal steps involve evaluating their stability under various conditions and unraveling the kinetics of drug release. These evaluations offer crucial information on the robustness of the agents and their ability to deliver drugs effectively over time.
Figure 4 strategically highlights pivotal stages in the development and assessment of nanoantibacterial agents, with a primary focus on in vitro testing and preclinical studies. The in vitro testing phase involves a thorough evaluation of the antibacterial activity of the nanoagent against a diverse range of bacterial strains. Various in vitro antibacterial methods are used, including determining the minimum inhibitory concentration (MIC), minimum bactericidal concentration (MBC), time-kill kinetics, checkerboard assays, biofilm disruption assays, and disk diffusion assays. Additionally, in vitro studies are conducted to assess the agent's safety and efficacy against bacterial strains.
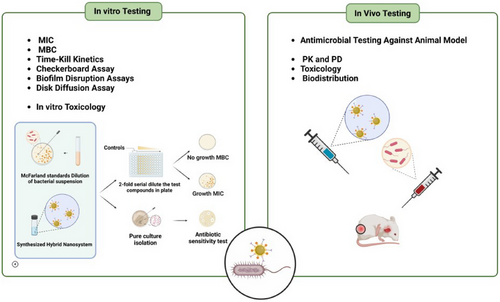
- Toxicity assessment: To ensure the safety of the agent, cytotoxicity tests using mammalian cell lines are performed. This step is vital in assessing the potential side effects of the agent.
- Pharmacokinetics (PK) and pharmacodynamics (PD): The analysis of PK and PD profiles helps understand absorption, distribution, metabolism, and excretion, correlating these factors with antibacterial efficacy.
- Biodistribution: Investigating the distribution of NPs within the body provides insights into accumulation and potential long-term effects. While this step is essential, the primary emphasis remains on in vitro and preclinical assessments, acknowledging the key role they play in the developmental process.
- Toxicology: Comprehensive toxicology studies, covering acute and chronic toxicity, genotoxicity, and carcinogenicity, are imperative.
To further delve into the intricate features of these nanosytems, we will now shift our focus to the controlled release mechanisms employed in their design.
Figure 5 represents the advanced features of nanoantibacterial agents, highlighting stimuli-responsive drug release and the intricate mechanisms underlying their action against bacteria.
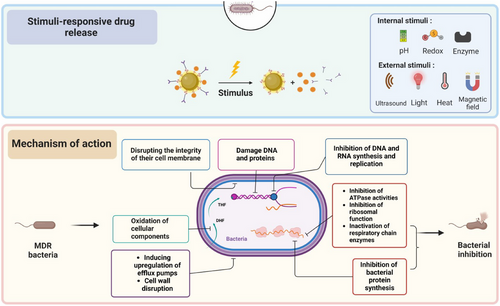
- Stimuli-responsive drug release: They can be intricately designed to release their antibacterial components in response to specific stimuli, either internal (e.g., pH changes) or external (e.g., heat, ultrasound, light, magnetism). This controlled drug release enhances precision in targeting bacteria, significantly improving the efficacy of the agent.
- Mechanism of action: Understanding how nanoantibacterial agents interact with bacteria and their components is vital. This figure explores the mechanisms by which these agents inhibit bacterial growth, addressing key molecular and cellular targets.
3.3 Classification of nanohybrid systems
Hybrid nanosystems are typically composed of inorganic and organic components, each having distinct characteristics that determine their practical applications and characterization methods (Figure 6).

Hybrid nanosystems blend a variety of materials, blurring the traditional lines between organic and inorganic components. The term “hybrid” encompasses various combinations, such as organic–organic, inorganic–inorganic, or organic–inorganic configurations. In this discussion, we categorize these materials into “organic building blocks” and “inorganic building blocks” for clarity. This categorization helps us to present and understand research papers based on their primary focus, without diminishing the overall importance of hybridization. Instead, it provides a practical framework to discuss the unique attributes and applications of these components in the treatment of MDR bacteria.
Inorganic building blocks commonly utilized include metals like gold nanoparticles (AuNPs) and iron oxide nanoparticles (IONPs), respectively, as well as other compounds such as zinc oxide nanoparticles (ZONPs) and calcium phosphate nanoparticles (CPNPs). Porous structures like mesoporous silica nanoparticles (MSNs) and metal–organic frameworks (MOFs) are also part of the inorganic building blocks. However, organic building blocks consist of polymers, copolymers, lipids, dendrimers, and isolated cell membranes. Additionally, there are carbon derivatives such as graphite oxide (GO), fullerene (C60), carbon nanotubes (CNTs), and graphene quantum dots (GQDs) that exhibit properties resembling both organic and inorganic systems. Consequently, these carbon-based materials are included in the classification because they can function as organic building blocks. The combination of these diverse materials has led to the development of a wide range of hybrid nanosystems aimed at addressing challenges encountered in the field of biomedical research. To better understand the landscape of NP choices and their prevalence in this critical area of biomedical research, we present Figure 7. This figure provides a frequency distribution diagram, offering information on the number of studies employing various types of MDR bacteria NPs considered for the antibacterial test. The data presented in Figure 7 exemplify the diverse applications of nanomaterials, showcasing their versatility in combating antibiotic resistance among MDR bacteria through innovative strategies.
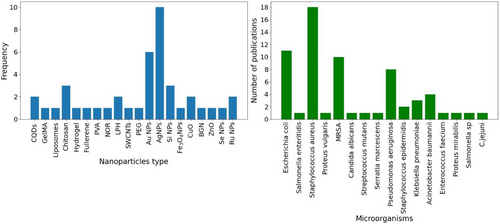
In our review, we categorized the selected articles based on the components utilized by each research group in their respective studies.
4 INORGANIC BUILDING BLOCKS
This section includes research papers under the “inorganic building blocks” category, which encompasses studies primarily centered around the utilization of inorganic nanosubstances in the development of hybrid nanosystems for MDR bacteria applications. It is essential to note that while some papers may involve a combination of both inorganic and organic nanosubstances in their methodologies, they are classified here based on their predominant focus on inorganic materials.
Inorganic building blocks encompass a wide range of materials, such as metal NPs, metal oxides, quantum dots (GQDs and CQDs not considered in this category), and other nanoscale structures.9 These NPs can exhibit enhanced antibacterial activity by disrupting bacterial membranes, generating reactive oxygen species, or interfering with bacterial signaling pathways. They offer capabilities in terms of controlled drug release, pH-responsive behavior, and photothermal effects, enabling precise and targeted treatment of MDR bacteria.10 Quantum dots, with their unique optical and electronic properties, have also found utility in hybrid nanosystems.11 They possess the potential for highly sensitive and specific bacterial imaging, facilitating the early diagnosis and monitoring of MDR infections. Furthermore, other nanoscale structures, such as mesoporous materials or layered nanomaterials, contribute to the controlled release of antimicrobial agents, enhancing the therapeutic effectiveness of the hybrid nanosystems.
Throughout this section, we will explore the diverse applications and contributions of these inorganic building blocks in hybrid nanosystems for MDR bacteria applications. Both gold and silver possess intrinsic antimicrobial properties, making them effective against a wide range of bacteria. AuNPs exhibit excellent stability and biocompatibility, while silver nanoparticles (AgNPs) exhibit strong antibacterial activity.
When combined in a hybrid nanosystem, these NPs synergistically interact, leading to enhanced antimicrobial effects. The hybrid nanosystems can disrupt the bacterial cell membrane, induce oxidative stress, and inhibit crucial cellular processes, ultimately leading to bacterial death.
Functionalization with biomolecules, such as peptides or antibodies, can further enhance their targeting ability toward specific bacterial strains or biofilms associated with MDR infections.
Although gold- and silver-based hybrid nanosystems have been extensively studied and hold great promise in addressing MDR bacteria, it is essential to recognize the significance of other hybrid nanomaterials. For example, hybrid nanosystems incorporating materials such as ZONPs, CPNPs, and MSNs offer unique attributes that can be tailored for specific applications. ZONPs, for instance, have demonstrated antimicrobial properties and are explored for their effectiveness against bacterial strains.12 CPNPs, on the other hand, are known for their biocompatibility and potential as drug delivery carriers, making them versatile candidates for controlled antibiotic release.13 MSNs, characterized by their porous structure, provide an ideal platform for loading and releasing antibacterial agents with precision, allowing for localized treatment.14
4.1 Gold-based materials
The potential of antimicrobial peptides (AMPs) and their self-assembly on gold nanodots was examined as a strategy to fight against MDR bacteria and aid wound healing. The process of AMPs’ self-assembling on the surface of gold nanodots was investigated. Through this process, peptides bind to gold nanodots, creating a functionalized nanostructure that significantly increases the stability and antimicrobial efficacy of the peptides. These resulting self-assembled antimicrobial peptide–gold nanodot structures were found to show potent antimicrobial properties against MDR (S. aureus, Salmonella enteritidis, Escherichia coli, Proteus vulgaris, MRSA).15 The nanostructures are engineered to specifically attack and break down bacterial membranes, which results in the death of bacterial cells. Furthermore, potential applications of these self-assembled nanostructures in wound healing were probed. The peptide–gold nanodot complexes can function as protective agents against bacterial infections in wounds, a major issue when dealing with MDR bacteria. In addition, these nanostructures might promote wound healing by fostering an environment conducive to tissue regeneration.16 However, it is important to acknowledge that one of the weaknesses of this research is the lack of comparison with the existing antimicrobial agents or treatments. Comparative studies would help evaluate the relative effectiveness of this new material.
A hybrid nanosystem, uniting gold–silver (Au/Ag) nanocages with AMPs and hyaluronic acid (HA) has been developed.17 The synthesis meticulously follows a process that begins with the seeding growth method for AgNPs and then employs galvanic replacement reactions for Au/Ag nanocage assembly. The characterization reveals a stable size of 128 nm for HA-P(Au/Ag), showcasing remarkable photothermal conversion efficiency and a loading capacity of 28.4 µg of AMPs per 12 µg of Au/Ag NCs. Focused on MDR Acinetobacter baumannii (MDRAB), in vitro and in vivo assessments elucidate the disruption of bacterial membranes by AMP-modified Au/Ag nanocages. Safety evaluations exhibit minimal cytotoxicity and negligible impact on kidney and liver functions. While showcasing potential against MDR infections, the study advocates for long-term safety assessments and stability optimizations for future clinical applications.
Comparative analyses underscore the importance of addressing limitations and challenges to elevate the nanosystem's therapeutic efficacy. Another study delving into AuNPs’ potential against MDR bacteria emphasized an eco-friendly synthesis.18 The study extensively investigated d-amino acid-based surfactants, coupled with gold and silica NPs, employing environmentally conscious methods for synthesizing these potent antimicrobial agents. Specifically, the research highlighted a surfactant-free emulsion technique for AuNPs synthesis and a specialized process integrating silica gel and eco-friendly surfactants to produce silica nanoparticles (SiNPs). Mechanisms of action highlighted the disruption of bacterial membranes through micelle formation. Additionally, the study addressed challenges, limitations, and optimization needs, particularly targeting enhanced efficacy against Gram-negative bacteria, emphasizing the potential of these agents in combating antimicrobial resistance. Moreover, it detailed the creation of D-Arginine ethyl ester hydrochloride SiNP (D-LAE-SiNP-Si) complexes using silicone resin. The efficacy measures demonstrated promising antimicrobial activity against E. coli, S. aureus, Candida albicans, and Streptococcus mutans. The study revealed low cytotoxicity and enhanced stability in its safety and toxicity assessment. In vitro experiments validated the nanocomposites’ antimicrobial activity by exhibiting growth inhibition or bactericidal effects across diverse bacterial strains.
In conjunction with above-mentioned discussion, a study was conducted utilizing not only AuNPs but also AgNPs.19 The research delved into hybrid nanosystem synthesis methods employing chemical techniques with citrate as a stabilizing agent for AuNPs and AgNPs, utilizing chemical hybridization through covalent bond formation. A comparative assessment was designed, evaluating these NPs coupled with fluoroquinolone antibiotics against MDR E. coli and S. aureus strains. The comparison revealed a consistent preference for AgNPs over gold; AuNPs exhibited minimal control over both E. coli and MRSA strains, showing slight effects only at a 100 mL concentration, while AgNPs displayed substantial efficacy even at minimal concentrations against E. coli. Efficacy varied across different drugs; ciprofloxacin and levofloxacin performed well even at lower AgNP concentrations, whereas norfloxacin, ofloxacin, and pefloxacin required relatively higher AgNP concentrations. Against S. aureus, all drug hybrids showed moderate effects, highlighting the need for increased NP concentrations for effective growth inhibition. The investigation successfully demonstrated the binding of fluoroquinolone drugs to both AuNPs and AgNPs during identification and characterization studies. Safety evaluations indicated no observed toxicity linked to the citrate stabilizing agent. In vitro assays employing E. coli and MRSA elucidated mechanisms of action, showcasing AgNPs’ roles in collapsing membrane potential, inhibiting ATPase activities, and ribosomal binding inhibition. However, notable limitations were evident, including a lack of comprehensive characterization techniques for the structure of hybrid NPs. Moreover, the absence of statistical analysis and constraints in establishing the full significance of AuNPs were identified as challenges within the study.
Moreover, in another research study, a hybrid nanosystem synthesis method involving chemical reduction techniques to fabricate 4,6-diamino-2-pyrimidinethiol-functionalized AuNPs (DAPT-Au NPs) integrated within a silk fibroin (SF) matrix for wound dressing applications has been presented.20 This innovative approach was followed by a well-structured study design encompassing various stages. Initial efficacy measures involved assessing MDR E. coli strains through MIC measurements and disk diffusion assays, subsequently employing zone of inhibition assessments and bacterial viability assays to demonstrate the potent antibacterial efficacy of the composite film. Identification and characterization were meticulously conducted, confirming successful synthesis and characterization of DAPT-Au NPs and DAPT-Au-SF mixed-matrix membranes (MMMs). Safety evaluations, focusing on cytotoxicity assessments, revealed remarkable biocompatibility, even at the highest concentrations of DAPT-Au NPs. The in vitro assessments utilized bacterial strains, exhibiting effective antibacterial action by collapsing membrane potential, inhibiting ATPase activities, and ribosomal binding, resulting in inhibited bacterial growth. Lastly, challenges and limitations were acknowledged, highlighting weak x-ray diffraction signals due to low NP concentration and the challenge in confirming DAPT-Au NP presence in MMMs, emphasizing the need for optimization in characterization techniques in future studies.
The last study in the section concerning AuNP-based systems initially elaborates synthesis methods, detailing a citrate reduction approach for AuNPs, precise encapsulation protocols for rifampicin (RF) and isoniazid (INH) into chitosan-grafted pyromellitic dianhydride–cysteine (AuNPs, RF, INH, CS-g-PMDA-CYS), and polymer synthesis.21 Efficacy measures evaluate the system against MDR Serratia marcescens, a cause of urinary tract infections, employing MIC determination, swarming motility inhibition, prodigiosin pigment inhibition, biofilm analysis, and Caenorhabditis elegans infection models. Identification and characterization methods, along with drug release patterns, confirm structural, morphological, drug delivery characteristics, and loading capacities of 9.02 % for RF and 13.12 % for INH. It demonstrates effective antimicrobial properties against MDR S. marcescens, inhibiting biofilm development and reducing virulence factors. Additionally, in infection models using C. elegans, the system led to an increased survival rate during S. marcescens infection. Notably, the findings suggest the potential of this developed system as a drug carrier and delivery system for targeting MDR bacteria, particularly S. marcescens, associated with urinary tract infections. The study highlights challenges including addressing drug resistance and improving drug delivery efficacy. However, the absence of cytotoxicity data underscores the need for additional toxicity studies, emphasizing the imperative for a more comprehensive investigation of this system.
4.2 Silver-based materials
In the realm of antimicrobial materials, silver-based compounds and NPs have emerged as promising candidates due to their well-documented antibacterial properties. This subsection delves into the world of “silver-based materials,” exploring the diverse range of silver-based compounds, NPs, and their applications in combating MDR bacteria. These materials have shown great potential in various biomedical and environmental applications, providing innovative solutions in the ongoing battle against MDR bacteria.
Considering silver-based hybrid nanosystems, the potential of hybrid photosensitizers to effectively combat MRSA without depending on antibiotics is unveiled in a comprehensive study of Ahmadov et al.22 The study's objective was to develop hybrid photosensitizers by combining AgNPs and photosensitizing molecules using chemical hybridization via covalent bond formation, further detailed with the inclusion of (trihydroxysilyl)propyl methylphosphonate (TMHP) for stability. The efficacy measures demonstrated the notable impact of these hybrid photosensitizers against MDR bacteria strains, particularly MRSA. Utilizing photodynamic inactivation assays and various assessment methods such as MIC determination, inhibition assays, and infection models, the study revealed an enhanced killing efficacy of up to six orders of magnitude against MRSA. Safety and toxicity assessments underscored the critical need for more comprehensive toxicity studies to definitively determine safety.
Both in vitro assays involving MRSA under light illumination conditions and in vivo C. elegans infection models further validated the efficacy of these hybrid photosensitizers. The antibacterial mechanisms of action hinged on the generation of singlet oxygen, showcasing their potential in neutralizing MRSA without reliance on antibiotics. However, the study emphasized the challenges in addressing antibiotic resistance, improving drug delivery efficacy, and the urgent need for further toxicity studies, highlighting crucial areas that require attention and optimization for future research endeavors.
The following study was conducted to investigate the potential of AgNPs in a hybrid nanosystem; however, it stands alone in our review as the singular paper constructing an inorganic–inorganic hybrid system.23 The incorporation of carbenicillin in this experiment introduces the potential for an inorganic–organic composite within the context of AgNPs. This paper comprehensively describes the synthesis of a hybrid nanosystem comprising Ag/Ag2O NPs through extracellular synthesis with Kitasatospora albolonga. The process spanned 72 h and involved the reduction of silver nitrate, yielding a distinct reddish-brown solution indicative of NP formation. Various characterization techniques confirmed the presence of the hybrid NPs, revealing their unique core–shell structure: an Ag2O shell that envelopes an Ag core. Although explicit loading capacity details were omitted, the study emphasized the hybrid nature of the synthesized material. The study design encompassed detailed characterization techniques alongside antimicrobial assessments. In particular, synthesized NPs exhibited considerable antimicrobial efficacy against MDR P. aeruginosa, particularly in synergy with carbenicillin. Carbenicillin disks infused with AgNPs showed the most promising synergistic inhibition. Although the evaluations were primarily in vitro, no explicit mention was made regarding in vivo models. Antibacterial mechanisms predominantly involved adherence to microbial cell walls, disrupting cell integrity and inducing membrane deformation. However, specific details on safety, toxicity, or nuanced findings in this context remained absent. The study underscored the challenges of addressing high MIC values, potentially originating from MDR strain resistance, thus stressing the urgency of optimized strategies to combat MDR.
Eco-friendly processes for the fabrication of stable and biocompatible silver-lignin NPs have also been explored.24 One synthesis method, using lignin as both a reducing agent and capping agent, embodies a sustainable approach that yields AgLNPs of enhanced stability and reduced environmental impact. Methodologically, the study encompasses a comprehensive design involving diverse assessment techniques revealing crucial details regarding AgLNP's size, stability, surface activity, and silver confirmation. The investigation rigorously assesses antibacterial efficacy against MDR strains— S. aureus, S. epidermidis, Pseudomonas aeruginosa, Klebsiella pneumoniae, and A. baumannii —through MIC and MBC assays, effectively inhibiting bacterial growth while preserving human cell viability. Mechanically, it delineates the antibacterial action of AgLNPs, illustrating bacterial cell disruption, penetration, and efflux pump induction, leading to upregulation of efflux-related membrane proteins and downregulation of unrelated membrane proteins. Safety evaluations reveal selective cytotoxicity across diverse cell lines, emphasizing the need for optimization strategies to address cytotoxicity variations and potential biofilm-related resistance.
The challenges encompass bacterial resistance mechanisms, toxicity concerns, and the need for broader efficacy. Importantly, comparison results with conventional antibiotics or other NP formulations showcase the superior efficacy and reduced cytotoxicity of AgLNPs, underlining their potential as an alternative therapeutic option.
Another study emphasizes this eco-friendly and cost-effective approach in synthesizing a hybrid nanosystem, centered on the biosynthesis of AgNPs derived from Bacillus horikoshii AJM-A1 supernatant and their subsequent conjugation with α-amylase, resulting in bioconjugates with improved antibacterial properties.25 It demonstrated strong inhibition against MDR biofilms, particularly those of K. pneumoniae and MRSA. The system effectively prevented biofilm formation and dispersed mature biofilms, underscoring its potential for clinical applications in managing biofilm-associated infections. The study demonstrates substantial activity at concentrations ranging from 25 to 800 µg/mL. Notably, the outcomes reveal significant inhibition of bacterial growth and biofilm formation in a dose-dependent manner. Identification and characterization methods confirmed the successful synthesis of irregular and spherical AgNPs (average size: 13.47 ± 12 nm) via supernatant reduction of AgNO3, highlighting high elemental silver content and specific functional groups involved in AgNP reduction, indicating enhanced loading capacity. However, the study lacks explicit safety assessments. Despite successful outcomes, identified limitations include size-dependent effects on biofilm reduction and observed aggregation limiting effectiveness against established biofilms. Thus, the study outlines the necessity for optimization strategies, such as surface functionalization, to enhance stability and cellular uptake. The antibacterial mechanisms of the hybrid system involve the disruption of the cell wall, oxidation of cellular components, enzyme inactivation, ROS generation, and enhanced membrane permeability, resulting in increased enzymatic activity and effective biofilm inhibition. The in vitro studies highlight the efficacy of the hybrid system, while in vivo assessments are not explicitly addressed. In comparison, the hybrid system demonstrates superior antibacterial and antibiofilm effects compared to AgNPs alone, signifying its potential for biomedical applications in the treatment of biofilm-related infections. The identified challenges of the study encompass a lack of quantitative data and specific measurements related to AgNP properties, underscoring the need for optimization and quantitative investigations to fortify the study's robustness. In accordance with the findings, a study explores the dual properties of antibacterial and anticancer efficacy through a comprehensive examination of the synthesis and characterization of a hybrid nanosystem.26 This nanosystem was synthesized by employing Rosa damascenes extract for AgNP production and subsequent conjugation with chitosan. The focus remains on evaluating the efficacy against MDR bacteria, specifically E. coli and MRSA strains. The combination of Cefotaxime-CS-AgNPs demonstrated significant antimicrobial activity, reducing MIC compared to cefotaxime alone. Notably, Cefotaxime-CS-AgNPs showed no cytotoxic effects on normal cells, even at high concentrations, establishing an IC50 for human RPE-1 normal cells and MCF-7 breast cancer cell lines. Key findings included upregulation of pro-apoptotic genes (p53, p21, and Bax) and concurrent downregulation of the antiapoptotic gene Bcl-2 in cancer cell lines after 48 hours of treatment at a concentration of 24 µg/mL, suggesting potential apoptotic pathways triggered by cefotaxime-CS-AgNP. Moreover, although not explicitly addressed, the nanosystem demonstrated promising anticancer potential due to selective cytotoxicity against cancer cells, highlighting its dual efficacy against MDR bacteria and its potential in cancer treatment. The study also revealed differential cytotoxic effects of AgNPs on normal and cancer cells. Cefotaxime-CS-AgNPs exhibited lower toxicity in normal cells and moderate effects in cancer cells at higher concentrations. Antibacterial mechanisms suggested the accumulation of cefotaxime-CS-AgNPs within bacterial cells, potentially disrupting membrane functions and preserving cefotaxime's efficacy against β-lactamases. Identified challenges emphasized the necessity for robust statistical analyses. The findings of this study highlight its translational relevance in revitalizing the effectiveness of older antibiotics against resistant bacterial strains.
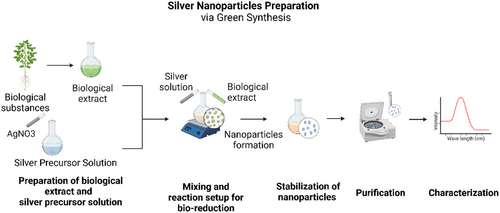
The findings of this subsection are summarized in the Table 1. This table provides a comprehensive overview of the key studies and their findings on silver-based materials, highlighting the significant contributions and varied approaches in the field. The detailed summarization of silver-based materials is justified as a result of their diverse approaches, extensive research, and complex data. Additionally, Figure 8 provides a schematic representation of the green synthesis process for AgNPs, illustrating the key steps such as the preparation of biological extracts, silver precursor solutions, NP formation, purification, and characterization.
| Systems components | Synthesis and hybridization method | Main advantages | Study ID |
|---|---|---|---|
| AgNPs + AuNPs, HA, AMPs | Chemical synthesis methods “Covalent Bond Formation” | Controlled synthesis, functionalization, photothermal conversion, antibacterial properties, biocompatibility | 17 |
| AgNPs/AuNPs + Fluoroquinolone | Chemical reduction and “Covalent Bond Formation” | Enhanced antibacterial properties, stability, controlled synthesis, silver significantly outperforms gold | 19 |
| Silver core, mesoporous silica shell (Ag@MS) + Photosensitizing molecules (HPIX) | Chemical reduction and “Covalent Bond Formation” | Antibacterial activity, singlet oxygen generation, broad excitation profile, antibiotic-free approach, good stability | 22) |
| AgNPs + Lignin molecules | Chemical reduction and chemical hybridization | Size and stability control, high surface activity, broad-spectrum antibacterial activity, selective cytotoxicity for cancer therapy, immunomodulatory effects | 24 |
| AgNPs + α-amylase | Biological synthesis and bioconjugation | Eco-friendly synthesis, cost-effective method, antibacterial and antibiofilm properties, biocompatibility | 25 |
| AgNPs + Chitosan Cefotaxime | Biosynthesis synthesis and chemical conjugation | Antimicrobial activity, controlled drug release, enhanced cytotoxicity against cancer cells, apoptosis induction, stability against β-Lactamases, biocompatibility | 26 |
|
AgNPs + mSiO2 TSD SWCNTs |
Chemical reduction process and a template-based synthesis, chemical hybridization, and covalent bond formation | Antibacterial activity and potential for treating drug-resistant infections, improved dispersion, wound healing, biocompatibility, biosafety | 27 |
| AgNPs + GQDs | Hydrothermal solvothermal, synthesis, and chemical hybridization | Antibacterial activity, wound healing, biocompatibility green synthesis, biosafety | 28 |
4.3 Iron oxide
In hybrid nanosystems, IONPs are widely used as inorganic materials. Both maghemite (γ − Fe2O3) and magnetite (Fe3O4), which are different forms of IONP, exhibit biocompatibility and low toxicity, which makes them attractive for various biomedical applications.29 In the synthesis of IONPs, various methods such as chemical, physical, and biological approaches have been utilized. Among these methods, chemical synthesis has gained popularity and is widely employed. The synthesis of IONPs can be easily achieved through the basic coprecipitation of iron salts.
By controlling pH during the synthesis process, it is possible to achieve variations in the particle size of IONPs.30 IONPs possess inherent antimicrobial properties, allowing them to directly target and inhibit the growth of MDR bacteria. It can serve as carriers for antibacterial agents, allowing targeted and controlled release of therapeutic compounds specifically to MDR bacteria, enhancing their efficacy. Moreover, the surface of IONPs can be modified with targeting ligands, antibodies, or other molecules, allowing specific recognition and binding to MDR bacteria, enhancing their selective delivery.31
A hybrid nanosystem comprising Fe3O4 NPs coated with polyethylene glycol (PEG) and loaded with gentamicin (Gen) showed efficacy against MDR bacterial strains— S. epidermidis, P. mirabilis, and A. baumannii.32 This comprehensive evaluation revealed a noteworthy reduction in biofilm formation and bacterial death at 100 µg/mL. The nanocomposite's synthesis process—employing co-precipitation, PEG coating, and Gen loading—tailored the material for optimized antibacterial efficacy. The Fe3O4NPs+PEG+Gen composite exhibited notable improvements in antibacterial effectiveness, which holds substantial promise for advanced therapeutic applications. The in vitro models underscored Fe3O4 NPs’ potential in disrupting biofilms and generating reactive oxygen species to inhibit bacterial growth. However, in vivo models showed variable efficiencies that highlight the need for further optimization for a greater applicability of therapeutics. Acknowledging limitations in the study design, the research emphasized the necessity for extensive toxicity evaluations and deeper exploration of in vivo models to faithfully replicate clinical scenarios and ensure a comprehensive understanding of the hybrid nanosystem's safety profile.
4.4 Copper oxide nanoparticles
Copper oxide (CuO) NPs have gained significant interest due to their antimicrobial and biocidal features, making them suitable for numerous biomedical applications33, 34 CuO is a semiconductor metal that has distinctive optical, electrical, and magnetic characteristics, enabling its utilization in various fields. CuO NPs can effectively inhibit the growth of various MDR bacteria, including both gram-negative and gram-positive strains. They disrupt the bacterial cell membrane and generate ROS that induce oxidative stress, leading to bacterial cell death, and can enhance the antibacterial properties of various substances when combined, creating more effective treatments for drug-resistant bacteria.35 This combination demonstrates improved efficacy against MDR bacteria, with examples including CuO NPs paired with antibiotics, polymers, or other NPs.36, 37 The NPs can act as carriers for antimicrobial drugs, allowing targeted delivery to bacterial infection sites.
Based on the earlier discussion, a hybrid nanosystem comprising chitosan-based copper oxide (CH-CuO) NPs was synthesized using Trianthema portulacastrum extract, employing an eco-friendly approach.38 As we mentioned before, this method not only ensures eco-friendliness by avoiding hazardous chemicals but also infuses potential biological activities from the plant extract into the NPs. Identification techniques confirmed their poly-dispersed spherical morphology with an average size of 17.37 nm, further affirming successful synthesis and revealing significant loading capacity. The extensive antibacterial assessments against MDR strains (E. coli, P. aeruginosa, Enterococcus faecium, S. aureus) showed substantial antibacterial effects, exhibiting the highest inhibition zone against E. coli while showcasing notable actions against biofilm formation, reducing extracellular polymeric substances (EPS), and altering cell surface hydrophobicity across all bacterial isolates, albeit with the lowest inhibition zone noted against S. aureus. Additionally, the NPs demonstrated noteworthy antioxidant properties with an 81 ± 4.32 % radical scavenging activity and moderate inhibition of alpha amylase, suggesting potential antidiabetic qualities. Mechanisms of action involved interactions between NPs and cell membranes, impacting nutrient flow, permeability, DNA and protein interaction, and generating reactive oxygen species, ultimately causing cellular damage and restricting growth. Despite robust antibacterial efficacy, the study highlighted challenges like the absence of in vivo studies and limitations in directly applying in vitro findings, emphasizing the necessity for further research to validate practical applications, especially in exploring safety, broader applications, toxicity, and stability.
4.5 Other inorganic nanomaterials
In addition to the specific subcategories of inorganic building blocks, such as gold based and silver based, this section describes papers that feature unique inorganic materials not explicitly covered by the predefined subcategories.
Following the above-mentioned guideline, a compelling hybrid nanosystem that addresses MDR bacterial infections has been proposed.39 The in vivo studies displayed significant efficacy in treating infections and promoting wound healing in mice, with accelerated recovery, reduced inflammation, and tissue regeneration. Comparative analysis within the study revealed the nanosystem's superior activity against MDR strains compared to individual components or other treatments, emphasizing its potential in combating drug-resistant bacteria and biofilm disruptions. Safety evaluations confirmed low toxicity and favorable biocompatibility, suggesting clinical utility, although optimization of ascorbic acid (AA) concentrations is needed. The AA@Ru@HA-molybdenum disulfide (MoS2) hybrid nanosystem, comprising ruthenium nanoparticles (RuNPs), hyaluronic acid (HA), MoS2, and AA, was designed for precise targeted antibacterial drug delivery. Its synthesis involved MoS2 NP synthesis, HA conjugation, RuNP loading, and integration of AA. Characterization methods confirmed good structure and demonstrated controlled drug release, uniformity, and efficacy against MDR strains. Diverse antibacterial tests, including confocal laser scanning microscopy (CLSM) and in vitro assays, highlighted biofilm disruption and in vivo wound healing acceleration. Demonstrating robust antibacterial action, wound healing, and low toxicity, the nanosystem shows promising clinical potential against drug-resistant infections, emphasizing chemo-photothermal therapy's precision targeting.
The antibacterial effects primarily rely on photothermal therapy facilitated by RuNPs inducing localized hyperthermia, damaging bacterial cells’ membranes and structures, and leading to bacterial cell death. Additionally, incorporating AA potentially acts as a prodrug, undergoing chemical transformations or enzymatic reactions, releasing active antimicrobial components interfering with crucial bacterial functions, and ensuring bacterial cell death. This dual-action mechanism ensures precise bacterial eradication, potentially minimizing resistance and effectiveness against drug-resistant strains and biofilms.
In summary, inorganic building blocks exhibit unique properties that make them valuable for combating MDR bacteria. AuNPs and AgNPs provide high stability and intrinsic antibacterial activity, while IONPs combine drug delivery and magnetic responsiveness. CuO NPs show effectiveness against both Gram-positive and Gram-negative bacteria. However, these materials face challenges, including high costs, potential toxicity, and limited in vivo efficiency due to aggregation and oxidative stress. Future research should focus on eco-friendly synthesis methods, biocompatibility enhancement through surface modifications, and long-term toxicity studies to facilitate their clinical application.
5 ORGANIC BUILDING BLOCKS
Organic building blocks encompass a wide array of materials, including biodegradable polymers, lipids, and organic NPs.5 These organic elements offer exceptional versatility and biocompatibility, making them instrumental in tailoring nanoscale platforms for targeted drug delivery, controlled release, and specific interactions with bacterial cells.
The categorization is guided by the pivotal role of organic materials in the methodologies and applications expounded upon in each paper. As previously noted, it is essential to reiterate that while some papers may integrate both inorganic and organic nanosubstances, their inclusion within this category is contingent upon their principal focus on organic building blocks.
5.1 Liposomes
Hybrnanosystems may rely on liposomes as the main organic component. Liposomes are spherical bilayers of lipids enclosing an aqueous cavity. Lipid-based nanosystems demonstrate several advantageous characteristics, including biocompatibility, self-assembly ability, straightforward synthesis, substantial loading capacity, and the capability to deliver substances across cell membranes.40 Although there are a variety of methods for obtaining liposomes, Figure 9 shows the classic film hydration method as an illustrative example.
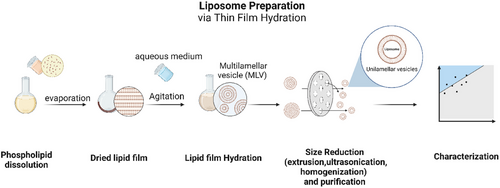
Liposomes are commonly created using a mixture of lipids to regulate the zeta potential, which determines the surface charge. Cationic liposomes exhibit improved cellular uptake, but their stability in serum is compromised. On the other hand, neutral or anionic liposomes show limited uptake and their stability in serum can vary.41
Commencing this subcategory, a study investigated the development of a novel gastroresistant antimicrobial formulation employing pectin (PCT) and liposomes (LIP) as carriers, referred to as PCT@LIP/NOR.42 The synthesis of this hybrid nanosystem capitalized on the pH disparity between these components (PCT and LIP) to effectively load norfloxacin (NOR). The study's design demonstrated 180 days of physicochemical stability at 4°C, alongside revealing superior loading capacity for NOR. Evaluation against MDR bacterial strains, including Salmonella sp., P, aeruginosa, E. coli, and Campylobacter jejuni, utilized MIC50 testing for antibacterial assessment, conducted both in vitro and in vivo using a chicken embryo model. The results showcased heightened antimicrobial activity of PCT@LIP/NOR compared to commercial NOR and liposomal suspensions and indicated their potential in oral antimicrobial formulations. Mechanisms of action unveiled an enhanced interaction with bacterial cell walls, aligning with potential mechanisms like oxidation of cellular components, penetration, and interaction with DNA and proteins, thus amplifying the release and antimicrobial effectiveness of NOR. Safety evaluations in chicken embryos exhibited no toxicity, corroborated by biochemical marker analysis, highlighting the formulation's potential for clinical applications. Moreover, the hybrid pectin-liposomal formulation, PCT@LIP/NOR, exhibited antioxidant properties, indicated by elevated total antioxidant capacity and thiol group levels compared to free norfloxacin and negative controls. This effect is likely attributed to the presence of α-tocopherol within the liposomes, presenting an added advantage in shielding cellular components from oxidative stress. However, limitations primarily revolved around the absence of human clinical data for this specific hybrid system, signifying the necessity for further validation.
In a similar study, organic-organic hybrid systems have been explored.43 This study presents an in-depth analysis of a novel pH-responsive lipid-polymer hybrid NP system known as VCM-GAPAH-LPHNPs, comprising polyallylamine hydrochloride (PAH), oleic acid (OA), vancomycin (VCM), and 18β-glycyrrhetinic acid (GA). The synthesis used the microemulsion technique, ensuring stable pH-responsive behavior for enhanced drug release at acidic infection sites. The study meticulously characterized the system, highlighting favorable attributes such as particle size, surface charge switch, stability, and encapsulation efficiency. Antibacterial efficacy evaluations included tests against MRSA through crystal violet assays, time-kill kinetics, and biofilm disruption assays. Within VCM-GAPAH-LPHNPs, vancomycin targets bacterial cell wall synthesis, binding to the growing peptide chain, while 18β-glycyrrhetinic acid disrupts bacterial cell membrane synthesis and DNA replication, collectively boosting antibacterial effectiveness, notably in acidic infection sites. In other words, their programmable response to acidic bacterial microenvironments underscores their clinical utility. Encapsulation of these agents within NPs likely optimizes drug release, enabling targeted action against bacteria, including antibiotic-resistant strains like MRSA. In vitro studies demonstrated a remarkable 16-fold improvement in antibacterial activity against MRSA compared to the raw drug, confirming the synergistic action of vancomycin and 18β-glycyrrhetinic acid. Safety assessments, encompassing cytotoxicity and haemolysis studies, affirmed biosafety within tested concentrations. The findings emphasized the system's boosted antibacterial effectiveness. However, identified limitations highlighted observed cytotoxicity at higher concentrations, underscoring the crucial need for refining targeting and biosafety aspects for clinical translation. Moreover, conducting in vivo studies is essential to thoroughly evaluate the system's performance and validate its potential in a biological context.
5.2 Carbon-based materials
Carbon-based derivatives, including GO, C60, CNTs, and GQDs, exhibit properties that resemble both organic and inorganic systems. These materials have shown promising applications in combating MDR bacteria when incorporated into hybrid nanosystems. GO can be utilized as a coating on various surfaces, such as medical devices, to inhibit bacterial growth and prevent biofilm formation.44 Additionally, it can be functionalized with antibacterial agents or combined with antibiotics to enhance their efficacy against MDR bacteria.45 Moreover, fullerene derivatives, CNTs, and GQDs are promising agents against MDR bacteria.46 They serve multifaceted roles, acting as carriers for antibiotics and enhancing the efficacy of existing antimicrobial agents. These nanomaterials possess distinct capabilities, including membrane disruption and targeted drug delivery, making them invaluable in combating drug-resistant pathogens.
A simplified and eco-friendly method was utilized to synthesize a carbon-based nanosystem that exhibits remarkable photostability and fluorescence stability, ensuring reliability and longevity for various uses.47 The study introduced a formulation termed p-CQD/WS2/n-CQD/GelMA, which integrates positively charged carbon quantum dots (p-CQDs), tungsten disulfide nanosheets (WS2 NSs), and nitrogen-doped carbon quantum dots (n-CQDs) within gelatin methacryloyl (GelMA) hydrogel scaffolds. The hybrid synthesis method involved a sequential process of individually preparing these components before integrating them into the GelMA matrix, harnessing the distinct properties of each material.
In vitro assessments with human mesenchymal stem cells (hMSCs) and in vivo evaluations using MRSA-infected mice models for skin wounds and craniotomy defects were conducted, highlighting the compound's potential in biomedical applications. The antibacterial mechanism of action, attributed to robust electrostatic and hydrophobic interactions between p-CQDs and bacterial membranes, induced membrane damage, leading to bacterial death. This effective mechanism displayed superiority against the tested MDR strains, contributing to the system's robust antibacterial properties. Safety evaluations showcased excellent biocompatibility with minimal cytotoxicity and no observed oxidative stress.
Addressing challenges such as antibiotic-induced issues and inadequate osseointegration, proposed strategies involving surface charge regulation aim to enhance antibacterial and osteogenic activities within the synthesized biomaterials. Overall, this hybrid nanosystem exhibited superior antibacterial efficacy against MDR strains compared to conventional antibiotics or single-component systems.
Other study utilized SWCNTs, notably extends its impact beyond antibacterial properties to promote wound healing.27 This study outlines the chemical synthesis processes and hybridization, ingeniously combining Single-Walled Carbon Nanotubes (SWCNTs), N-[3-(Trimethoxysilyl) propyl] ethylene diamine (TSD), and Mesoporous Silica (mSiO2) to create the distinctive SWCNTs@mSiO2-TSD-Ag nanoplatform. This amalgamation tactically blends SWCNTs, Ag NPs, and mSiO2, resulting in a potent antibacterial nanoplatform, confirmed by characterizations affirming its unique structure and properties. Encapsulating SWCNTs within mesoporous silica significantly enhances their dispersibility and distribution, contributing to heightened antibacterial activity against drug-resistant strains like E. coli and S. aureus. Moreover, this nanoplatform not only shows improved dispersion and wound healing capabilities but also exhibits remarkable biocompatibility and biosafety. Mechanistic insights reveal the nanoplatform's efficacy, damaging bacterial cell membranes, swiftly releasing silver ions, and inhibiting cellular components. Safety evaluations affirm its cellular compatibility even at higher concentrations, endorsing its potential application. The findings suggest improved wound healing, reduced infection severity, and controlled bacterial growth upon nanoplatform application. Its efficacy against MDR bacterial strains surpasses standard antibiotics and commercial silver nanoparticles leveraging a dual antibacterial mechanism, proving more effective than traditional treatments. However, the initial tendency of SWCNTs to aggregate might present a limitation, potentially affecting the uniform dispersion and consistent antibacterial activity within the nanoplatform.
In a similar study, insights were drawn from an extensive study concentrating on a hybrid nanosystem, GQDs@Ag, synthesized via an eco-friendly method.28 The synthesis encompassed hydrothermal treatment of GQDs employing starch as a precursor, subsequent addition of AgNO3 and trisodium citrate, and ensuing reduction with NaBH4 for AgNP coating. The resultant nanocomposites displayed superior dispersion, stability, and heightened antibacterial activity against MDR bacterial strains, namely E. coli, S. aureus, and MRSA. Antibacterial assessments encompassed disk diffusion assay and antimicrobial testing against animal models. While exhibiting its capability to disrupt bacterial membranes, induce reactive oxygen species, and cause cellular damage, the study used in vitro and in vivo models to evaluate efficacy, revealing substantial enhancements in antibacterial action, safety, and wound healing potential of GQDs@Ag. The nanocomposites demonstrated good biocompatibility and low cytotoxicity, assessed through hemolysis and cytotoxicity assays, platelet aggregation assay, blood parameters (AST, ALT), and organ histology. Notably, the GQDs@Ag-Band-Aid treatment significantly improved wound closure and healing over a 10-day period compared to alternative treatments like PBS-Band-Aid, GQDs-Band-Aid, and AgNPs-Band-Aid. Although the study showcased promising results, its limitations included a narrow focus on MRSA, limiting broader bacterial applicability, and short-term in vivo experiments for wound healing, supporting their use in clinical wound treatments.
The development of tetraamino fullerene and benzothiadiazole fluorophore hybrid nanoparticles (TAFE-TBAc NPs) through a noncovalent conjugation process have been also explored.48 The synthesis method resulted in nanoparticles displaying enhanced stability and biocompatibility.
Characterization techniques confirmed the integrity of the hybrid nanoparticles. Loading capacity assessment revealed efficient energy transfer between the components, leading to improved photodynamic therapy (PDT) efficacy, with 80.8 % fluorescence quenching and an 18-fold increase in reactive oxygen species (ROS) yield. Antibacterial assessment against MRSA, involved colony-forming units (CFU) for bacterial growth, visible light exposure, staining techniques (crystal violet staining), and imaging (3D CLSM), demonstrated a 99.9 % elimination rate and biofilm inhibition upon visible light exposure. The in vitro studies on COS 7 cells and red blood cells displayed slight cytotoxicity at high concentrations with negligible erythrocyte destruction. The antibacterial mechanism involved ROS generation, particularly hydroxyl radicals and singlet oxygen, leading to irreversible damage and bacterial death. Challenges encompassed concentration-dependent cytotoxicity and selectivity toward Gram-positive bacteria. Optimization opportunities include broadening the antibacterial spectrum and minimizing concentration-related toxicity, despite the absence of in vivo results.
5.3 Polymers
Polymers are components within the broader category of organic building materials. Polymers can be designed to possess antimicrobial activity, biocompatibility, and stability.6 Several strategies have been explored in the development of polymer-based hybrid nanosystems.6 One approach involves incorporating antimicrobial agents, such as antibiotics or antimicrobial peptides, into polymer nanoparticles.49 These nanoparticles can improve the drug's stability, enhance its penetration into bacterial cells, and potentially overcome resistance mechanisms.
Another strategy is to incorporate nanoparticles with inherent antimicrobial properties, such as silver or copper NPs, into polymer matrices.50 These hybrid nanosystems can exhibit synergistic effects, combining the antimicrobial properties of the nanoparticles with the controlled release capabilities and biocompatibility of the polymers. Furthermore, researchers have explored the use of stimuli-responsive polymers in hybrid nanosystems.51, 52 These polymers can undergo specific changes in response to external stimuli, such as pH, temperature, or light, which can trigger the release of antimicrobial agents at the site of infection or within bacterial cells, enhancing their efficacy against MDR bacteria.
The following study describes a multifunctional hybrid system that shows antibacterial effects, tumor therapy, and wound healing, utilizing both in vitro and in vivo models, thereby establishing a strong foundation for diverse applications of the bioactive hybrid nanosystem.53 The study amalgamated monodispersed polydopamine functionalized bioactive glass nanoparticles (BGN@PDA) into an antibacterial F127-ϵ-Poly-L-lysine hydrogel. The synthesis methods meticulously delineated the formation of this multifunctional hydrogel, emphasizing its components and processes. Characterization employed a spectrum of techniques to unveil structural and compositional specifics. The results demonstrated strong antibacterial effectiveness against MDR strains, validated through in vitro and in vivo assays using standard agar plates and antimicrobial tests on animal models, highlighting the pronounced efficacy of the BGN@PDA-infused hydrogel against S. aureus, MRSA, and E. coli. While the assessment of antibacterial mechanisms highlighted the hybrid nanosystem's effectiveness, specific safety and toxicity assessments were not explicitly detailed, albeit mentioning biocompatibility and low toxicity. The multifunctional nature of the described nanosystem in the study heralds promising several capacities. In addition to its antibacterial activity against controls like BGN@PDA alone and ampicillin, the nanosystem showcased promising wound healing effects compared to controls such as FCE hydrogel and commercial dressings like 3 M, particularly in a full-thickness skin wound model in mice. A limitation lies in the absence of exploration through in vivo models or in vitro assays to observe its impact on wound closure rates, tissue regeneration, or anti-inflammatory responses, which could accentuate its applicability in expediting more efficient wound healing processes. Additionally, acquiring long-term data on the gel's effects could offer valuable insights.
Another study elucidates the production and characterization of biodegradable nanofibrous hybrid membranes integrating polyvinyl alcohol (PVA) with ZONPs and CuO NPs.54 The primary focus was evaluating these membranes’ potential against two critical challenges: combating anti-COVID-19 effects and MDR bacteria. The outcomes demonstrated that ZnO-loaded nanofibers exhibited robust antiviral activity against SARS-CoV-2 and superior antibacterial efficacy against various MDR bacterial strains compared to CuO-loaded nanofibers. The synthesis involved electrospinning as the physical method to integrate these nanoparticles into the PVA matrix. The study revealed ZnO-loaded nanofibers’ superiority, particularly in combating MDR bacteria strains such as MRSA, S. aureus, K. pneumoniae, MRSE, and P. aeruginosa. Evaluation methods included disk diffusion assays and quantitative techniques to determine antimicrobial activities. In vitro analyses formed the experimental core, focusing on SEM, TEM, and FTIR analyses for nanofiber characterization and nanoparticle loading capacity determination. The antibacterial mechanisms highlighted ZnO's smaller particle size and concentration, leading to enhanced efficacy compared to CuO, delineating their distinct actions on bacterial cells. ZnO nanoparticles exhibited enhanced antibacterial efficacy due to their smaller size and concentration, inducing oxidative stress, damaging cell walls, and increasing membrane permeability, leading to cell rupture and subsequent death. CuO nanoparticles functioned by associating with thiol groups in proteins, penetrating cells, and releasing copper ions, resulting in cell envelope component rupture and protein denaturation. Safety assessments indicated no cytotoxicity with ZnO nanoparticles and underscored their effective wound healing properties. The findings indicated ZnO nanoparticles’ biocompatibility, efficient antibacterial properties, and wound healing potential, suggesting their safe biomedical applications. Challenges and limitations focused on nanoparticle distribution and concentration optimization for maximal antibacterial efficacy. This comprehensive analysis enriches the understanding of these nanofibrous membranes’ interactions, offering valuable insights for future research and practical applications in combating MDR bacteria, however, some limitations exist.
The research lacks detailed analysis of dose-response relationships, comparative kinetics between ZnO and CuO formulations. While emphasizing ZnO's superiority, the study could explore synergistic effects and long-term biocompatibility beyond wound healing.
In a similar study, a hybrid nanosystem was synthesized using the ionic gelation technique, comprising chitosan and imipenem as components to create CS-IPM-NCS.55 The study methodically elucidated the efficacy measures against MDRAB, employing diverse antibacterial testing methods such as the disk diffusion assay, MIC, time-kill kinetics, and biofilm disruption assays. The research demonstrated successful inhibition and control of MDRAB growth, as well as biofilm disruption, highlighting the promising outcome of the nanocarrier system loaded with imipenem. Identification and characterization techniques including SEM, AFM, FTIR, and zeta potential analysis affirmed high encapsulation efficiency, stable particle size, and optimal morphology. Loading capacity assessment revealed efficient drug encapsulation within the nanocarrier system. The antibacterial mechanism of action involved the nano-carrier system targeting bacterial cell membranes through cationic-anionic interactions, leading to cellular component disruption and subsequent cell death. Comparative analysis with other results showcased the challenges of MDRAB strain resistance and biofilm permeability limitations, underscoring the need for optimization strategies, particularly in enhancing antibiotic penetration within biofilms. The potential of the research work could indicate the possibility of this hybrid nanosystem becoming a viable alternative or complement to existing antibiotics, considering its stability and effectiveness against MDR bacteria. The current work's limitations include the absence of in vivo studies and comprehensive toxicity assessments, crucial for understanding the nano-antibiotic system's safety profile within living organisms.
In summary, organic building blocks, including liposomes, polymers, and carbon-based materials, offer significant advantages in drug delivery and antimicrobial applications due to their versatility and ability to be tailored for targeted therapies. Liposomes efficiently show an excellent biocompatibility and allow encapsulation of various types of drugs, while polymers allow for controlled, stimuli-responsive drug release. Carbon-based materials exhibit mechanical strength and antimicrobial activity. However, challenges such as stability issues in liposomes, inconsistent drug release in polymers, and aggregation or cytotoxicity in carbon-based materials need to be addressed. Improvements in formulation stability, achievement of precise drug release mechanisms, and expanded biocompatibility studies could enhance their therapeutic potential.
6 OTHER HYBRID NANOSYSTEMS
In addition to the specific organic and inorganic components discussed earlier, hybrid nanosystems have showcased remarkable versatility and potential in the treatment of infectious diseases caused by MDR bacteria. This section delves into the applications of the components that were not included in previous sections due to their specific nature. Throughout this subsection, we will consider various organic and inorganic systems, highlighting their distinct properties, mechanisms of action, and potential clinical applications.
The study from Hassan et al.56 pioneered a novel synthesis of a hybrid nanosystem by crafting a pH-responsive zwitterionic lipid (OLA). This involved dissolving chitosan (CHs) in acetic acid, adjusting pH, and precisely combining tripolyphosphate (TPP), OLA, and the drug vancomycin (VM) to achieve ionic complexation, resulting in the successful formulation of VM–OLA–LPHVs. Evaluation against MDR bacteria, particularly MRSA, utilized diverse methodologies, including broth microdilution, time-kill assays, and biofilm reduction. These were complemented by MIC determination, fractional inhibitory concentration (FIC) indices, DNA/protein assays, flow cytometry, and fluorescence microscopy to dissect antibacterial mechanisms. The in vitro studies encompassed multiple cell lines, while in vivo assessments involved an MRSA skin infection model in BALB/c mice, revealing effective bacterial membrane disruption, increased permeability, and substantial biofilm reduction. Biocompatibility assays confirmed formulation safety with tested cell lines.
Administration of the antibiotic amikacin using carrier erythrocytes elicited a sustained release effect, with an increase in the plasma half-life and in the area under the curve of the antibiotic. Amikacin-loaded erythrocytes functionalized with glutaraldehyde demonstrated important changes in the accumulation of the antibiotic in organs of the reticulo-endothelial system (RES).57
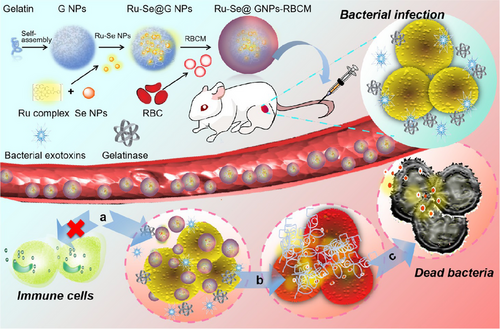
The last investigated study focuses on synthesizing the hybrid nanosystem Ru-Se@GNP-RBCM, incorporating ruthenium and selenium (Ru–Se NPs) within red blood cell membrane (RBCM) to enhance its antibacterial properties.8 The synthesis involved sequential steps, as depicted in Figure 10: preparing selenium nanoparticles, loading them with ruthenium, encapsulating them into gold nanoparticles, and modifying their surface with red blood cell membranes, possibly utilizing co-precipitation, chemical reduction, or a modified Stöber method. Characterization methods confirmed the nanosystem's morphology, size distribution, chemical composition, crystal structure, and drug loading/release behavior. Antibacterial tests showed a time-dependent reduction in survival rates of MDR bacteria strains (MRSA, E. coli, S. aureus, S. epidermidis, and P. aeruginosa) when incubated with various nanosystem concentrations. The nanosystem's characterization highlighted immune evasion, exotoxin neutralization, enzyme-responsive drug release, biocompatibility, and stealth properties, demonstrating bacterial membrane damage, ROS production, and disrupted integrity, leading to non-resistant bacterial death. Mechanistically, it engages reduced immune clearance, efficient exotoxin neutralization by RBCM, gelatinase-triggered degradation of GNPs at infection sites, and subsequent release of Ru-Se NPs for bacterial destruction. Furthermore, its fluorescence imaging capabilities enable accurate infection treatment monitoring. In vitro assays and in vivo mouse models confirmed antibacterial efficacy and safety, revealing minimal hemolysis, high cell viability, normal blood indicators, and no adverse organ effects. However, process optimization remains unaddressed. In addition, comparative results displayed enhanced antibacterial efficacy against multiple drug-resistant strains, reduced hemolytic activity caused by bacterial exotoxins, accelerated drug release triggered by gelatinase, and stronger effects against Gram-positive compared to Gram-negative bacteria. Notably, in vivo studies exhibited superior wound healing and efficient site-specific accumulation, validating the nanosystem's potential for targeted therapy and real-time infection detection.
The reviewed studies encompass a wide range of experimental setups, including both in vivo and in vitro investigations. One of the key trends observed across these studies is the combination of organic components or organic and inorganic components. Organic components, such as antimicrobial peptides, surfactants, and natural polymers, are skillfully integrated with inorganic nanoparticles like gold, silver, copper oxide, and others. This strategic combination has led to synergistic effects, as evident from the enhanced antimicrobial activity observed in the hybrid nanosystems compared to their individual counterparts. The diverse modes of action, such as disruption of bacterial membranes, inhibition of cellular components, and oxidation of cellular materials, have contributed to the potent bactericidal capabilities of these nanomaterials. Such findings open up new avenues for combating bacterial infections, particularly those involving MDR organisms that pose significant challenges to conventional antibiotic therapies.
Beyond their antimicrobial prowess, the reviewed studies shed light on the biocompatibility and safety of the hybrid nanosystems, which are vital considerations for their potential translation into clinical applications. The demonstrated biocompatibility is a promising indicator that these nanosystems could be incorporated into medical interventions without causing undue harm to the host. Furthermore, several investigations explored additional benefits, such as wound healing properties and controlled drug delivery capabilities of the hybrid nanosystems. These discoveries reveal the multifaceted potential of these nanosystems, offering opportunities for diverse biomedical applications beyond their primary antimicrobial role. The integration of environmentally friendly synthesis methods in some studies underscores the growing emphasis on sustainable nanotechnology practices. Such green synthesis techniques contribute to reducing the environmental impact of nanomaterial production and highlight researchers’ commitment to ethical and sustainable scientific development.
Collective evidence showcases the importance of interdisciplinary efforts in leveraging the unique properties of organic and inorganic components to address the global challenge of antimicrobial resistance. As promising candidates for combating drug-resistant bacterial strains, these hybrid nanosystems have potential as a complementary strategy to existing antibiotic therapies. Furthermore, the versatility of these nanomaterials, demonstrated by their wound healing properties and controlled drug delivery potential, presents new avenues for further exploration and innovation in nanomedicine. However, it is important to acknowledge that more comprehensive studies and safety assessments are essential before their translation into clinical practice. With continued research and development, these hybrid nanosystems may contribute significantly to the future landscape of antimicrobial treatments, offering novel solutions to combat bacterial infections and potentially revolutionize the field of infectious disease management.
Table 2 summarizes some illustrative examples of hybrid nanosytems of different types and their advantages.
| Systems components | Synthesis and hybridization method | Main advantages | Study ID |
|---|---|---|---|
| Pectin + Liposomes+ NOR | Sol–gel method and nanoencapsulation | Enhanced drug loading, extended drug release, potent antimicrobial activity, biocompatibility, stability, antioxidant properties | 44 |
| VCM + GA + LPHNPs | Self-assembly microemulsion and physical hybridization | Enhanced antibacterial activity, targeted drug delivery, sustained drug release, biocompatibility, stability | 45 |
| TAFE + TBAc | Multi-step chemical synthesis and physical hybridization | Selective antibacterial activity, enhanced PDT, efficiency, biocompatibility and stability, biosafety, biofilm inhibition | 50 |
| Chitosan + imipenem | Ionic gelation technique and nanoencapsulation | Biofilm inhibition, stability, antibacterial activity | 57 |
| Antimicrobial peptides +SFT+ DT+ Au NPs | Chemical reduction and thiol reactions | Strong antimicrobial properties, minimal cytotoxicity, low hemolytic effects, wound healing | 17 |
| SF mixed-matrix membrane + DAPT-AuNPs | Chemical reduction and nanoencapsulation | Antibacterial activity, biocompatibility, enhanced mechanical properties, hydrophilicity and water absorption, wound healing, antibacterial dressing | 22 |
| CS-g-PMDA-CYS + RF + INH + AuNPs | Chemical reduction and covalent bond | Improved drug delivery, effective encapsulation, pH-responsive release, biofilm inhibition | 23 |
| VM+ LPHVs + OLA | Chemical ionic complexation and nanoencapsulation | Green and eco-friendly synthesis, high stability, optimal nanoparticle size, antibacterial activity, biocompatibility, stability | 58 |
| Gen + PEG + Fe3O4NPs | Co-precipitation and covalent bond | Enhanced antibacterial activity, broad-spectrum effectiveness biofilm inhibition, controlled antibiotic release, stability | 34 |
| Running TitleRunning Title3232Chitosan + CuO | Biological synthesis | Green synthesis and cost-effective, biocompatibility and biodegradability, antimicrobial activity, biofilm inhibition, antioxidant activity, antidiabetic potential, long-term stability, low toxicity, versatility | 40 |
| GelMA/CQDs + WS2 nanosheets (NSs) | Chemical microwave assisted synthesis and covalent bond formation | Green synthesis, tunable properties, promotion of bone regeneration, biocompatibility, photostability and fluorescence stability, antibacterial activity, photothermal performance, biocompatibility low toxicity, simplified preparation | 49 |
| PDA + F127-ϵ-Poly-L-lysine Hydrogel + BGN | Sol–gel method and biomimetic coatings /chemical hybridization | Multifunctionality: tumor therapy wound healing, infection control, injectable and self-healing, antibacterial activity, photothermal performance, biocompatibility, low toxicity, simplified preparation | 55 |
| Running TitleRunning Title3535Running TitleRunning Title3636PVA + CuO + ZnO | Physical synthesis electrospinning and physical hybridization electrospinning | Antiviral activity, antibacterial activity, biodegradability, no cytotoxicity, low cost, flexibility in metal oxide choice | 56 |
| GNPs + RBCM + Se NPs +Ru | Chemical reduction and template-based synthesis, biological hybridization and biomimetic Coatings chemical hybridization | Antibacterial properties immune evasion, exotoxin neutralization, enzyme-responsive drug release, multifaceted mechanisms, biocompatibility, biodistribution targeting, wound healing, safety and stability, economic, stealth property | 8 |
| HA + AA + Ru NPs +MoS2 nanosheets | Chemical reduction and chemical hybridization | Targeted drug delivery, synergistic effect, biofilm disruption, biocompatibility, wound healing | 41 |
In summary, hybrid nanosystems integrating organic and inorganic components provide synergistic benefits, offering tailored properties for specific bacterial targets and diverse infection scenarios. Despite their versatility and enhanced antimicrobial activity, these systems often involve complex synthesis processes that hinder scalability and lack long-term safety data. Simplifying synthesis methods, validating safety and efficacy through preclinical and clinical studies, and exploring new material combinations could significantly advance their practical application and clinical translation.
Moreover, as a key feature of nano-antibacterial systems, including organic, inorganic, and hybrid nanosystems, stimuli-responsive drug release mechanisms enable precise targeting and controlled therapeutic delivery. These mechanisms, categorized into internal, external, and multi-stimuli triggers, are summarized in Table 3, providing a comprehensive overview of the strategies employed throughout this review.
| Stimuli type | Mechanism | |
|---|---|---|
| Internal stimuli | pH responsive | Degradation of pH-sensitive coatings in acidic environments |
| Enzyme responsive | Degradation by bacterial gelatinase enzymes | |
| Redox responsive | Disulfide bond cleavage by elevated GSH levels | |
| External stimuli | Temperature responsive | Drug release by heat or swelling |
| Light responsive | ROS generation via photosensitizers | |
| Magnetic field responsive | Heating by magnetic nanoparticles | |
| Ultrasound responsive | Cavitation or mechanical disruption triggered by ultrasound waves | |
| Multistimuli | Combination of stimuli (e.g., pH and ROS response) | |
7 CONCLUSION
This review underscores the significant potential of hybrid nanosystems in combating MDR bacterial infections. Among the systems evaluated, silver-based nanosystems, particularly those combined with gold, stand out for their robust antimicrobial properties, stability, and biofilm-disruptive capabilities, with preclinical studies confirming their efficacy against MDR pathogens such as MRSA and E. coli. Clinical approval of silver-based wound dressings further highlights their translational feasibility.25, 26
Liposomal hybrid nanosystems, such as PCT@LIP/NOR, demonstrate superior drug delivery efficiency, prolonged stability, and enhanced efficacy against MDR strains compared to conventional antibiotics. The success of liposomal antibiotics in clinical trials further supports their potential for widespread use. Similarly, stimuli-responsive nanosystems, including enzyme-responsive and photothermal systems, offer precise drug delivery and antibacterial action, exemplified by systems like AA@Ru@HA-MoS2. These advanced platforms highlight opportunities for targeted infection control and wound healing.
To achieve successful clinical translation, rigorous preclinical and clinical evaluations focusing on safety, toxicity, and scalability are essential. Current clinical trials on liposomal antibiotics42, 43 and silver-based25, 26 formulations provide a roadmap for advancing these technologies, while further studies are needed to establish the utility of stimuli-responsive and carbon-based systems.
With continued innovation and interdisciplinary collaboration, hybrid nanosystems offer a transformative approach to managing MDR bacterial infections, providing effective and sustainable alternatives to traditional antibiotics and addressing the urgent threat of antimicrobial resistance.
ACKNOWLEDGMENTS
Figures in this manuscript were created using BioRender.com.
CONFLICT OF INTEREST STATEMENT
The authors declare no conflict of interest.
Biographies

Pegah Tabatabaei Tabrizi is a PhD student at the University of Salamanca. She holds an MSc in Pharmaceutical Chemistry and is currently specializing in Pharmaceutical Technology. Pegah's research focuses on antibacterial hybrid nanosystems, aiming to develop innovative solutions to combat infectious diseases caused by multidrug-resistant (MDR) bacteria.

José M. Lanao is a Professor of Pharmacy and Pharmaceutical Technology and the Head of Pharmaceutical R & D Laboratory at the University of Salamanca. He is also the Head of the Research Group about experimental and clinical pharmacokinetics at the Institute of Biomedical Research of Salamanca (IBSAL), Spain His research interest focuses on drug delivery; cell carriers; nanoparticles; pharmacokinetics; biodistribution; modelling and simulation; population pharmacokinetics; therapeutic drug monitoring; anti-infectives; and pharmaceutical development.

Carmen Gutierrez-Millan is an Associate Professor of Pharmacy and Pharmaceutical Technology in Pharmaceutical Sciences Department of the University of Salamanca. She is responsible for the Quality Assurance of Pharmaceutical R & D Laboratory at the University of Salamanca. Dr. Gutierrez-Millan has coauthored a variety of publications about Drug Delivery Systems. Her current research focuses on nanosystems derived from cellular membranes and nanoparticles.

Clara I. Colino holds a PhD in Pharmacy. Since 2005, she has been a Full Professor of Pharmacy and Pharmaceutical Technology at the University of Salamanca, where she teaches new strategies for drug administration. She has published diverse works in high-impact journals. Her research activity has focused on anti-infectious therapy mainly in drug biodistribution and nanosystems and biological carriers for their vectorization to macrophages.