From clinical observations to molecular insights: Decoding immune checkpoint inhibitors-induced prostatitis
Peng Luo, Ying Liu, and Lan Xu are joint authors. These authors have contributed equally to this work and share first authorship.
Abstract
Immune checkpoint inhibitors (ICIs) have dramatically transformed cancer treatment; however, their impact on the male reproductive system remains poorly understood. This study aims to elucidate the incidence, risk factors, and molecular mechanisms underlying ICI-associated prostatitis. We conducted an analysis of ICI-associated prostatitis utilizing the U.S. Food and Drug Administration Adverse Event Reporting System (FAERS) database and evaluated risk through the application of the reporting odds ratio. A mouse model of ICI treatment was developed, and subsequent transcriptomic changes in prostate tissue were investigated using high-throughput sequencing. Hematoxylin and eosin staining, immunohistochemistry, Von Frey testing, and enzyme-linked immunosorbent assay were employed to confirm ICI-induced prostatitis and further delineate its underlying mechanisms. Additionally, we investigated the therapeutic potential of specifically targeting interleukin-6 (IL-6) and extracellular signal-regulated kinase (ERK) signaling pathways. FAERS analysis demonstrated a statistically significant positive correlation between ICI treatment and prostatitis risk (p < .05). In the murine model, ICI treatment induced an elevated inflammatory response in prostate tissue, characterized by enhanced inflammatory cell infiltration and upregulated expression of IL-6 and tumor necrosis factor-alpha. Transcriptomic analysis revealed significant activation of multiple inflammation-related signaling pathways in prostate tissue following ICI treatment (false discovery rate < 0.05). Targeted inhibition of IL-6 or ERK signaling pathways significantly attenuated ICI-induced prostatitis symptoms, resulting in improved tissue pathology and decreased inflammatory factor expression (p < .01). This study delineates the characteristics and potential molecular mechanisms underlying ICI-associated prostatitis, thereby establishing a theoretical foundation for the development of prevention and treatment strategies. Targeted modulation of IL-6 and ERK signaling pathways may present novel therapeutic interventions for ICI-associated prostatitis, thus warranting further clinical validation.
Abbreviations
-
- ADE
-
- adverse drug event
-
- ADRs
-
- adverse drug reactions
-
- CD3
-
- cluster of differentiation 3
-
- CP
-
- chronic prostatitis
-
- CTLA-4
-
- cytotoxic T-lymphocyte-associated protein 4
-
- ELISA
-
- enzyme-linked immunosorbent assay
-
- ERK
-
- extracellular signal-regulated kinase
-
- FAERS
-
- FDA Adverse Event Reporting System
-
- FDA
-
- U.S. Food and Drug Administration
-
- H&E staining
-
- hematoxylin and eosin staining
-
- ICIs
-
- immune checkpoint inhibitors
-
- IHC
-
- immunohistochemistry
-
- IL-6
-
- interleukin-6
-
- irAEs
-
- immune-related adverse events
-
- irMRAEs
-
- ICI-related male reproductive adverse events
-
- PD-1
-
- programmed cell death protein 1
-
- PD-L1
-
- programmed cell death ligand 1
-
- PFS
-
- progression-free survival
-
- qPCR
-
- quantitative polymerase chain reaction
-
- ROR
-
- reporting odds ratio
-
- RT-qPCR
-
- real-time quantitative polymerase chain reaction
-
- ssGSEA
-
- single-sample Gene Set Enrichment Analysis
-
- TNF-α
-
- tumor necrosis factor-alpha
1 INTRODUCTION
Immune checkpoint inhibitors (ICIs) have increasingly emerged as a pivotal strategy in the treatment of various cancers.1 Currently, the most frequently targeted molecules by ICIs include programmed cell death protein 1 (PD-1) and its ligand (PD-L1), as well as cytotoxic T-lymphocyte-associated protein 4 (CTLA-4). Since the approval of the anti-CTLA-4 antibody (ipilimumab) for advanced melanoma treatment in 2011,2 numerous ICIs have subsequently received approval from the U.S. Food and Drug Administration (FDA) for the treatment of various cancer types.3 These agents include PD-1 inhibitors (such as pembrolizumab, cemiplimab, and nivolumab) and PD-L1 inhibitors (such as atezolizumab, avelumab, and durvalumab). These medications have significantly improved survival rates for patients with various cancers by enhancing T-cell-mediated anti-tumor immune responses.4 Studies have demonstrated that when ICIs are used as first-line cancer treatments, patients experience significantly prolonged progression-free survival (PFS) and a reduced incidence of treatment-related adverse events compared to conventional chemotherapy.5 Based on their remarkable safety and efficacy profiles, ICIs have been widely adopted in cancer treatment protocols under rigorous clinical monitoring. However, the various immune-related adverse events (irAEs) resulting from the non-specific effects of ICIs on the immune system have garnered increasing attention.6 The clinical manifestations of irAEs range widely, from mild inflammatory responses in endocrine, dermatological, and gastrointestinal systems7 to life-threatening conditions such as myocarditis and heart failure.8 These adverse events pose significant challenges to the clinical application of ICIs and necessitate careful patient monitoring and management.
Previous studies have predominantly focused on common adverse events associated with ICIs,9 with comparatively limited attention given to reproductive system-related adverse events. Yang et al. demonstrated that “reproductive system and breast disorders” were among the principal categories of adverse events in cancer patients aged 18‒64 years undergoing ICI therapy.10 Notably, existing studies indicate that ICI therapy may precipitate male reproductive system disorders.11 Brunet-Possenti et al. documented two cases of metastatic melanoma patients: one developed bilateral orchitis following ipilimumab‒nivolumab combination therapy,12 while the other manifested epididymo-orchitis subsequent to pembrolizumab treatment.13 Both cases presented with acute testicular pain and swelling. Analysis of the World Health Organization global database of individual case safety reports, VigiBase, spanning from 2011 to 2019, revealed a significant and disproportionate increase in the risk of hypogonadism in patients subsequent to ICI therapy. Of the 13 reported cases of hypogonadism, five were classified as secondary hypogonadism, and one as primary hypogonadism.14 At present, investigations into ICI-related male reproductive system adverse events are predominantly confined to case reports. Real-world studies have documented ICI-related prostatitis as an adverse event. An analysis of testicular autopsy tissue samples from 13 patients with metastatic melanoma demonstrated that six out of seven (86%) patients who underwent ICI therapy exhibited impaired spermatogenesis compared to six age-matched untreated controls.15 Previous studies have established that prostate diseases or prostatic dysfunction can impact sperm function.16 This finding implies that ICI therapy may influence sperm production through the induction of prostate inflammation. Prostatitis, a common disorder of the male reproductive system, may be intimately associated with ICI therapy. Nevertheless, there remains a paucity of comprehensive research and systematic reviews elucidating this association.
Prostatitis, particularly chronic prostatitis (CP), has been extensively demonstrated to significantly impair patients' quality of life. Patients frequently present with varying degrees of chronic pain, urinary symptoms (e.g., urinary frequency, urgency, and pain), and sexual dysfunction (e.g., decreased libido and erectile dysfunction).17 These persistent symptoms often lead to physical and mental exhaustion in patients, resulting in a significant decline in quality of life that extends to their professional and family life. Consequently, prostatitis treatment must extend beyond merely addressing inflammation to encompass improvements in patients' overall health and quality of life. Furthermore, prostatitis can potentially compromise male fertility. The prostate plays a crucial role in semen production, with its function being directly correlated to semen quality. CP is frequently associated with elevated leukocyte counts in semen, reduced sperm motility, and other semen quality abnormalities, thereby increasing the risk of infertility.18 Research has demonstrated a potential association between CP and male infertility, with heightened risk observed in cases of prostatitis induced by irAE. Thus, prostatitis treatment serves the dual purpose of alleviating immediate symptoms and improving male fertility, representing significant clinical importance.
Given the substantial clinical benefits of ICIs for cancer patients, a systematic analysis of the incidence, risk factors, and potential biological mechanisms of ICI-associated prostatitis is crucial for guiding clinical treatment decisions and improving patient outcomes. However, a comprehensive systematic analysis of ICI-associated prostatitis based on large-scale real-world data remains lacking. The FDA Adverse Event Reporting System (FAERS), one of the world's largest pharmacovigilance databases, contains extensive adverse event reports and patient information, offering valuable data support for validating and supplementing previous research findings. Furthermore, the risk factors for ICI-associated prostatitis and its impact on patient prognosis remain incompletely elucidated, and its molecular biological mechanisms warrant further in-depth investigation.
In this study, we utilize disproportionality analysis based on the FAERS database to systematically evaluate the incidence of prostatitis in male cancer patients receiving ICI treatment, and investigate its risk factors and impact on prognosis. Concurrently, we develop an ICI treatment mouse model and employ high-throughput sequencing technology to investigate molecular biological changes in prostate tissue, with the aim of providing experimental evidence for elucidating the pathogenesis of ICI-associated prostatitis. This study seeks to systematically investigate ICI-associated prostatitis from multiple perspectives by integrating real-world database analysis and animal model experiments, with the objective of providing robust evidence-based medical support for clinical practice.
2 METHODS
2.1 Signal mining of adverse reactions using real-world databases
This study analyzed data from the FAERS database, with data extracted from January 1, 2015, to December 31, 2023. The inclusion criteria comprised adverse drug event (ADE) reports from cancer patients, where the primary suspect drugs were PD-1 or PD-L1 inhibitors (e.g., nivolumab, pembrolizumab, cemiplimab, atezolizumab, avelumab, and durvalumab), CTLA-4 inhibitors (e.g., tremelimumab and ipilimumab), or standard chemotherapy agents. The FAERS database consists of six data tables: demographics, therapy, indications, drugs, reactions, and outcomes. These data tables were integrated using the Primary ID.19 In accordance with previous studies' definitions of ADE report duplication, this study excluded ADE reports that were identical in terms of sex, age, reporting country, date, adverse events, and drugs.20 Subjects were categorized into ICI and chemotherapy groups based on their treatment regimens. The FAERS database included 42,961 patients who received ICI treatment and 58,846 patients who underwent chemotherapy. This study designated ICIs as the target drugs and chemotherapy agents as controls. The reporting odds ratio (ROR) method was employed to assess the risk of ADEs following ICI treatment, using chemotherapy patients as the reference group. The ROR was specifically used to evaluate the risk of male reproductive system-related ADEs following ICI treatment, with chemotherapy patients serving as the reference group.21 A positive signal was defined as an ICI-associated risk with an ADE report count (a) ≥3 and the lower limit of the 95% confidence interval for ROR (ROR025) >1. Furthermore, subgroup analyses of ROR were performed based on age and ICI treatment regimens (e.g., PD-1 inhibitors, PD-L1 inhibitors, CTLA-4 inhibitors, and their combinations).
2.2 Effects of ICI treatment on the mice prostate
Eight-week-old male C57BL/6 mice (obtained from the Southern Model Organisms Center) were used for in vivo experiments. All animal experimental protocols were reviewed and approved by the Institutional Animal Care and Use Committee of the Zhujiang Hospital (no. LAEC-2023-222). The murine melanoma cell line B16-F10 was cultured in dulbecco's modified eagle medium (DMEM) supplemented with 10% fetal bovine serum and 1% penicillin‒streptomycin at 37°C in a 5% CO2 atmosphere. Cells were harvested in the logarithmic growth phase, resuspended in phosphate-buffered saline (PBS), and prepared at a concentration of 1 × 106 cells/100 µL with viability >95%. Male C57BL/6 mice (6‒8 weeks old) were housed under specific pathogen free (SPF) conditions, and 100 µL of the cell suspension was injected subcutaneously into the right flank. For therapeutic evaluation, tumor-bearing mice were randomly allocated into experimental groups. Prostate tissues were collected at the endpoint for further analysis, including histological and molecular evaluations. All procedures were approved by the institutional Animal Care and Use Committee, and experiments were conducted with at least three independent replicates. The mice were randomly divided into three experimental groups (n = 6 per group): (1) control group: received intraperitoneal injections of PBS (200 µL/mouse, biw) for 3 weeks. (2) Anti-PD-1 group: received intraperitoneal injections of monoclonal anti-mouse PD-1 antibody (200 µg/mouse, biw, BioXcell, clone RMP1-14, #BE0146) for 3 weeks. (3) Anti-PD-L1 group: received intraperitoneal injections of monoclonal anti-mouse PD-L1 antibody (200 µg/mouse, biw, BioXcell, clone 10F.9G2, #BE0101) for 3 weeks. Upon completion of the experimental period, mice were euthanized via intraperitoneal injection of an overdose of sodium pentobarbital, followed by prostate tissue harvesting for transcriptome sequencing analysis. Following quality control, sequence alignment, and gene expression quantification of the transcriptome sequencing data, single-sample Gene Set Enrichment Analysis (ssGSEA) was conducted to calculate the enrichment scores of signaling pathways for each sample. Subsequently, inter-group differential analysis was performed.22
2.3 Effects of anti-IL-6 or ERK inhibition on ICI-induced prostatitis
To investigate the effects of IL-6 inhibition and ERK pathway blockade on ICI-associated prostatitis, C57BL/6 tumor-bearing mice were randomly assigned to six experimental groups (n = 6 per group): (1) anti-PD-1 group: received intraperitoneal injections of monoclonal anti-mouse PD-1 antibody (200 µg/mouse, twice weekly) for 3 weeks. (2) Anti-PD-1 + anti-IL-6 group: received anti-PD-1 antibody as described above, with additional intraperitoneal injections of anti-mouse IL-6 antibody (200 µg/mouse, BioXcell, clone MP5-20F3, #BE0046) at days 16, 20, and 23. (3) Anti-PD-1 + ERK inhibitor group: received anti-PD-1 antibody as described above, with additional intraperitoneal injections of ERK inhibitor Ravoxertinib (50 mg/kg, Selleck Chemicals) at days 16, 20, and 23. (4) Anti-PD-L1 group: received intraperitoneal injections of monoclonal anti-mouse PD-L1 antibody (200 µg/mouse, twice weekly) for 3 weeks. (5) Anti-PD-L1 + anti-IL-6 group: received anti-PD-L1 antibody as described above, with additional administrations of anti-mouse IL-6 antibody following the same procedure as in group 2. (6) Anti-PD-L1 + ERK inhibitor group: received anti-PD-L1 antibody as described above, with additional administrations of ERK inhibitor Ravoxertinib following the same procedure as in group 3. Upon completion of the experimental period, mice were euthanized via intraperitoneal injection of an overdose of sodium pentobarbital. Subsequently, prostate tissues were harvested for hematoxylin and eosin (H&E) staining and immunohistochemical (IHC) analysis.
2.4 H&E staining and IHC analysis
After the experiment, the mice were euthanized via intraperitoneal injection of an overdose of pentobarbital sodium, and prostate tissues were subsequently extracted for H&E staining and IHC analysis. Conventional tissue embedding procedures and H&E staining were subsequently performed. The severity of prostatitis was assessed using a previously described four-tier scoring system.23 The specific scoring criteria were as follows: 0 points—no inflammation; 1 point—slight perivascular mononuclear cell infiltration; 2 points—moderate perivascular mononuclear cell infiltration; and 3 points—severe perivascular mononuclear cell infiltration with evident necrosis, hemorrhage, and extensive mononuclear cell infiltration within the tissue. Additionally, we conducted immunohistochemistry (IHC) staining on prostate tissues from the mice. The antibodies used were commercially available reagents, including anti-IL-6 antibody (R&D Systems, #AF-406-NA), anti-tumor necrosis factor-alpha (TNF-α) antibody (R&D Systems, #AF-410-NA), and anti-cluster of differentiation 3 (CD3) antibody (Cell Signaling Technology, #99940). The behavioral pain test and enzyme-linked immunosorbent assay (ELISA) utilized in this study are detailed in Supporting Information.
2.5 Quantitative polymerase chain reaction
Prostate tissue samples were collected, and the degree of inflammation among different groups was compared at the mRNA level using quantitative polymerase chain reaction (qPCR) technology. Primers for both target and reference genes were designed and synthesized by Beijing Qingke Biotechnology Co., Ltd. The primer sequences utilized in this study are detailed in Table S1.
2.6 Clinical sample collection
To further investigate the potential association between ICIs and prostatitis, we collected clinical samples from two groups of male cancer patients: (a) patients who had not received any prior radiotherapy or chemotherapy, and (b) patients who had undergone adjuvant treatment, including targeted therapy or immunotherapy. All patients met the following inclusion criteria: (1) presence of locally advanced tumors; (2) no prior history of prostate-related diseases, including prostatitis, benign prostatic hyperplasia (BPH), or prostate cancer, before receiving targeted therapy or immunotherapy; (3) abnormal prostate-specific antigen levels detected during follow-up after adjuvant treatment, with prostate biopsy confirming the absence of prostate cancer; and (4) no history of genetic disorders, infectious diseases, or other infections during the observation period. Baseline characteristics of the patients are summarized in Table S2. During the treatment period (weeks 0, 1, 2, 3, and 4), the National Institutes of Health Chronic Prostatitis Symptom Index (NIH-CPSI) scores were collected for each patient. The NIH-CPSI assessment encompasses domains for pain or discomfort, urinary symptoms, and quality-of-life impact. In addition, prostate tissue obtained from biopsies was subjected to H&E staining for histopathological evaluation, and inflammation scoring was performed accordingly. This study was approved by the institutional ethical review boards of the Zhujiang Hospital of the Southern Medical University (2024-KY-129-01). Written informed consent approved by the institutional ethics committee was obtained from each patient.
2.7 Statistical analysis
Logistic regression models, encompassing both univariate and multivariate analyses, were employed to evaluate the impact of prostatitis and other clinically relevant factors on cancer patient prognosis. Additionally, these models were used to analyze the association between various clinical factors and the occurrence of prostatitis in cancer patients. Cumulative distribution function curves were plotted to characterize the time distribution from initial drug administration to the onset of prostatitis. The Mann‒Whitney U-test was utilized to compare differences in median onset time of prostatitis among different gender and age groups. In the animal model study, the Mann‒Whitney U-test was employed to compare differences in ssGSEA scores for various signaling pathways between the PD-1 group and the control group, as well as between the PD-L1 group and the control group. In the experimental design, comparisons between two independent sample groups were conducted using unpaired Student's t-tests. Statistical significance was defined as p < .05 (two-tailed). Visual representations, including forest plots, bar charts, and box plots, were generated using the ggplot2 package. All data processing, statistical analyses, and graphical visualizations were conducted using R software (version 4.1).
3 RESULTS
3.1 Analysis of ICI-related male reproductive adverse events in the FAERS database
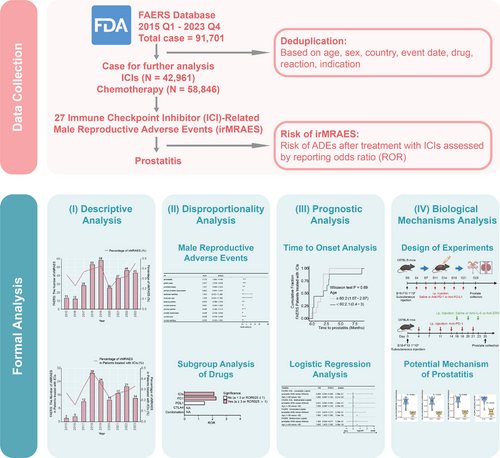
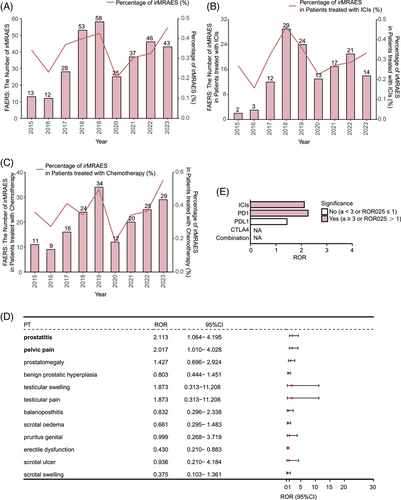
The analytical workflow of this study is illustrated in Figure 1. Following the deduplication of ADE reports for cancer patients receiving ICIs in the FAERS database, we analyzed the reports of target drug-related adverse events. In the FAERS database, we identified 27 types of ICI-related male reproductive adverse events (irMRAEs), including pelvic pain, balanoposthitis, scrotal erythema, genital pruritus, penile pain, scrotal ulcer, erectile dysfunction, genital rash, genital edema, scrotal dermatitis, prostatitis, genital ulceration, scrotal pain, genital erythema, penile swelling, scrotal swelling, perineal disorder, prostatic calculus, scrotal edema, BPH, prostatic disorder, testicular pain, prostatism, prostatomegaly, testicular swelling, prostatic calcification, and testicular mass. Our analysis of the FAERS data revealed that the frequency of irMRAEs in cancer patients treated with ICIs or chemotherapy fluctuated, but generally remained around 0.3% (Figure 2A). Among cancer patients treated with ICIs, the lowest proportion of irMRAEs was observed in 2016, at 0.158% (Figure 2B). For cancer patients receiving chemotherapy, the lowest proportion of irMRAEs was observed in 2020, at 0.184% (Figure 2C). Subsequently, we identified that only prostatitis and pelvic pain were statistically significant irMRAEs (Figure 2D; a ≥ 3, ROR025 > 1). We found that the ROR values for prostatitis varied across different ICI regimens, with the highest ROR value of 2.26 for anti-PD-1 therapy, while the ROR value for anti-PD-L1 therapy was 1.43 (Figure 2E).
3.2 Time to onset of ICI-related prostatitis and its impact on clinical prognosis
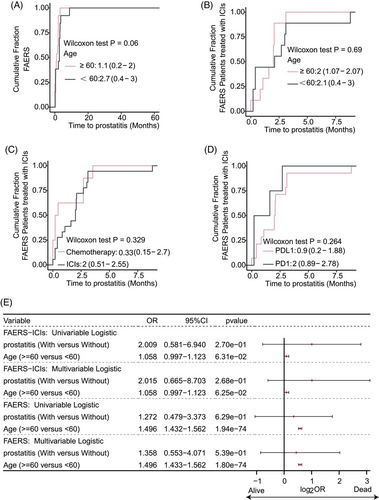
We analyzed the time interval from the initiation of ICIs or chemotherapy to the onset of prostatitis across different age groups. Our analysis revealed no significant difference in the time to onset of prostatitis between cancer patients aged <60 and ≥60 years (p > .05, Figure 3A). Similarly, among patients receiving ICI treatment, no significant difference was observed in the time to onset of prostatitis between the <60 and ≥60 years age groups (p > .05, Figure 3B). Furthermore, no statistically significant difference was observed in the time to onset of prostatitis between cancer patients receiving ICI treatment and those receiving chemotherapy (p > .05, Figure 3C). Likewise, different ICI treatment regimens (such as anti-PD-1 inhibitors and anti-PD-L1 inhibitors) demonstrated no significant difference in their impact on the time to onset of prostatitis in cancer patients (p > .05, Figure 3D). Univariate and multivariate logistic regression analyses revealed that the occurrence of prostatitis was not an independent risk factor for survival prognosis in cancer patients (p > .05, Figure 3E). Conversely, age ≥60 years was identified as an independent prognostic risk factor for cancer patients receiving ICI treatment or combination chemotherapy (p < .05, Figure 3E).
3.3 Development of ICI-related prostatitis may be associated with IL-6 or ERK pathway activation
The development of ICI-related prostatitis may be mediated through interleukin-6 (IL-6) and ERK pathway activation. The pathophysiological mechanisms underlying ICI-related prostatitis appear to involve the activation of IL-6 and ERK signaling pathways, which are well-established mediators of inflammatory responses. IL-6, an important inflammatory mediator, is involved in the inflammatory response, while the ERK signaling pathway is also implicated in this process.24, 25 Using murine models, we compared the activation levels of signaling pathways in prostate tissue between the ICI treatment group and the control group without ICI treatment. Our results demonstrated that, following ICI treatment, the activation level of the IL-6 response signaling pathway was significantly elevated compared to the control group (p < .05, Figure 4A). Subgroup analysis further revealed that both PD-1 and PD-L1 inhibitors led to a notable increase in IL-6 pathway activation (all p < .05, Figure 4B,C), highlighting the critical role of this cytokine in mediating prostate inflammation. Similarly, activation of the ERK1/2 cascade signaling pathway was significantly elevated in the ICI-treated group compared to the control group (p < .05, Figure 4D). Subgroup analysis indicated that this increase was specifically significant in the PD-L1 inhibitor group (p < .05, Figure 4E,F), suggesting its potential role in the inflammatory response.

3.4 ICI therapy has been observed to potentially induce prostatitis
In this study, 8-week-old male C57BL/6 tumor-bearing mice were used to establish a model of ICI-associated prostatitis through intraperitoneal administration of ICIs. The detailed protocol for the experimental model construction is illustrated in Figure 4G. To evaluate the effects of various treatment regimens on murine prostate tissue, multiple analytical methods were employed, including histopathological analysis of prostate tissue, ELISA, real-time qPCR (RT-qPCR), and nociceptive response tests. In comparison with the control group, both anti-PD-1 and anti-PD-L1 treatment groups exhibited significantly higher inflammation scores in prostate tissue, characterized by disrupted glandular architecture and extensive interstitial inflammatory cell infiltration (Figure 4H). The elevated levels of TNF-α and IL-6 demonstrated that ICI therapy induces a robust inflammatory response in prostate tissues, while the increased expression of CD3 highlights the role of T-cell-mediated immune activation in the development of ICI-related prostatitis (Figure 4H). Subsequent ELISA analysis revealed significantly higher concentrations of TNF-α and IL-6 in the prostate tissues of ICI-treated mice compared with those in the control group (Figure 4I). Correspondingly, RT-qPCR analysis corroborated significant upregulation of TNF-α, IL-6, and CD3 mRNA expression in both treatment groups (Figure 4J). Moreover, both treatment groups demonstrated a significant increase in pelvic stimulation response frequency compared with the control group, reflecting pronounced pelvic pain symptoms (Figure 4K). Collectively, these results provide compelling evidence that both anti-PD-1 and anti-PD-L1 therapies can induce prostatitis in murine models, characterized by inflammation and associated pain.
3.5 Blocking IL-6 or ERK signaling pathways significantly alleviates prostatitis induced by anti-PD-1 treatment
To further validate the roles of IL-6 and ERK signaling pathways in prostatitis associated with anti-PD-1 treatment, we established anti-PD-1 induction groups, anti-PD-1 combined with anti-IL-6 groups, and anti-PD-1 combined with anti-ERK intervention groups (Figure 5A). As illustrated in Figure 5B, blocking IL-6 or ERK signaling pathways significantly alleviated prostatitis symptoms in mice treated with anti-PD-1. Compared to the anti-PD-1 monotherapy group, both the anti-PD-1 combined with anti-IL-6 group and the anti-PD-1 combined with anti-ERK group exhibited significantly reduced inflammatory cell infiltration and expression levels of inflammation-related molecules, including TNF-α, IL-6, and CD3, in the prostate tissue of mice (Figure 5B). ELISA analysis demonstrated significantly reduced levels of TNF-α and IL-6 in both the anti-PD-1 combined with anti-IL-6 group and the anti-PD-1 combined with anti-ERK group (Figure 5C); RT-qPCR analysis further confirmed that the mRNA expression levels of TNF-α, IL-6, and CD3 were significantly downregulated in both the anti-PD-1 combined with anti-IL-6 group and the anti-PD-1 combined with anti-ERK group (Figure 5D). Furthermore, as illustrated in Figure 5E, the frequency of pain responses to tactile stimulation was significantly reduced in both the anti-PD-1 combined with anti-IL-6 group and the anti-PD-1 combined with anti-ERK group, indicating that blocking IL-6 or ERK signaling pathways significantly alleviates pelvic pain symptoms related to prostatitis induced by anti-PD-1 in mice.
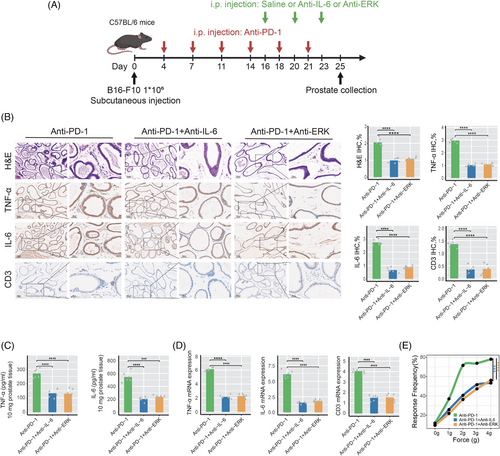
3.6 Blocking IL-6 or ERK signaling pathways significantly alleviates prostatitis induced by anti-PD-L1 treatment
To further validate the roles of IL-6 and ERK signaling pathways in prostatitis associated with anti-PD-L1 treatment, we established anti-PD-L1 induction groups, anti-PD-L1 combined with anti-IL-6 groups, and anti-PD-L1 combined with anti-ERK intervention groups (Figure 6A). As illustrated in Figure 6B, blocking IL-6 or ERK signaling pathways significantly alleviated prostatitis symptoms in mice treated with anti-PD-L1. Compared to the anti-PD-L1 monotherapy group, both the anti-PD-L1 combined with anti-IL-6 group and the anti-PD-L1 combined with anti-ERK group exhibited significantly reduced inflammatory cell infiltration and expression levels of inflammation-related molecules, including TNF-α, IL-6, and CD3, in the prostate tissue of mice (Figure 6B). ELISA analysis demonstrated significantly reduced levels of TNF-α and IL-6 in both the anti-PD-L1 combined with anti-IL-6 group and the anti-PD-L1 combined with anti-ERK group (Figure 6C); RT-qPCR analysis further confirmed that the mRNA expression levels of TNF-α, IL-6, and CD3 were significantly downregulated in both the anti-PD-L1 combined with anti-IL-6 group and the anti-PD-L1 combined with anti-ERK group (Figure 6D). Furthermore, as illustrated in Figure 6E, the frequency of pain responses to tactile stimulation was significantly reduced in both the anti-PD-L1 combined with anti-IL-6 group and the anti-PD-L1 combined with anti-ERK group, indicating that blocking IL-6 or ERK signaling pathways significantly alleviates pelvic pain symptoms related to prostatitis induced by anti-PD-L1 in mice.
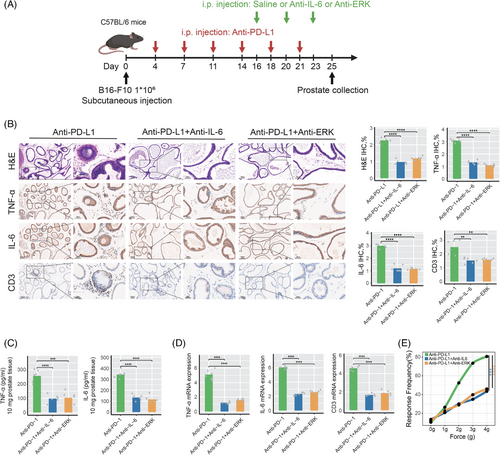
3.7 ICI-related prostatitis based on clinical validation
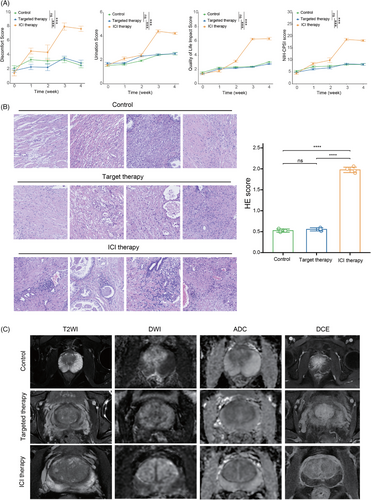
Compared to patients who did not receive any adjuvant therapy or those treated with targeted therapy, patients receiving ICIs exhibited a significant increase in pain scores, urinary symptom scores, quality-of-life scores, and NIH-CPSI scores 2 weeks after treatment (Figure 7A). Histological analysis using H&E staining revealed a higher abundance of inflammatory cell infiltration in the ICI-treated group, with the infiltrates predominantly localized in the prostatic stroma (Figure 7B). Furthermore, magnetic resonance imaging findings indicated significant prostate edema and enlargement in patients treated with ICIs (Figure 7C). These findings collectively provide strong evidence that ICIs can induce prostate-related inflammation in cancer patients.
4 DISCUSSION
Despite extensive reporting and research on irAEs associated with ICIs, comprehensive systematic studies on ICI-related prostatitis remain scarce. This study systematically investigated the incidence, risk factors, prognostic impact, and potential biological mechanisms of ICI-related prostatitis through a combination of real-world database analysis and animal model experiments. We conducted a large-scale retrospective study on adverse drug reactions (ADRs) in cancer patients undergoing ICI therapy from a pan-cancer perspective, utilizing the FAERS database. Our findings revealed a significantly higher incidence of prostatitis in male cancer patients undergoing ICI therapy compared to the control group not receiving ICI treatment. Additionally, using a mouse model, we compared the signaling pathway activation levels in prostate tissue between the ICI-treated group and the control group not receiving ICI treatment. Results demonstrated significantly elevated activity of ERK and IL-6 signaling pathways in the prostate tissue of ICI-treated mice. These findings provide a comprehensive characterization of the clinical features and potential biological mechanisms underlying the occurrence of prostatitis in male patients following ICI administration.
Our study demonstrated that there was no significant difference in the time to onset of prostatitis in ICI-treated patients between age subgroups and drug subgroups. Two potential mechanisms may explain this result. First, although immune responses among patients in different subgroups may differ in intensity or type, they likely share common immune activation pathways26 that lead to a similar time to onset of prostatitis, specifically through T-cell activation and cytokine release. Furthermore, from a pharmacokinetic perspective, minimal differences in drug absorption, distribution, metabolism, and excretion might exist among patient subgroups,27 particularly when administering identical ICIs. Consequently, this could result in comparable drug exposure levels across patients, leading to similar immune response kinetics. Furthermore, metabolic status has a potential impact on the immune response, and individual patient characteristics (e.g., obesity and systemic inflammatory state) are important factors influencing the onset and progression of irAEs. Recent research showed that obesity-driven systemic inflammation elevates baseline levels of IL-6 and PD-1, thereby priming immune cells for increased inflammatory responses.28 These findings suggest that factors such as obesity significantly influence the severity of irAEs, potentially leading to severe prostatitis in cancer patients receiving ICI therapy.
This study demonstrated significant activation of the ERK signaling pathway in the prostate tissue of mice receiving ICI treatment, indicating that the ERK signaling pathway might be involved in the pathogenesis of ICI-related prostatitis. ERK signaling pathway is a critical regulator of cell proliferation and differentiation.29 The mitogen-activated protein kinase (MAPK)/ERK signaling pathway, of which ERK is a component, is responsible for transmitting extracellular signals to the nucleus and functions as a central pathway regulating various cellular biological processes. In physiological conditions, this pathway is precisely regulated to maintain tissue homeostasis. Extensive research has demonstrated that the ERK signaling pathway plays a crucial role in inflammatory responses,30 and its dysregulation may result in tissue damage. For instance, Zhu et al. reported that inhibition of ERK signaling could reduce oxidative stress and inflammatory responses, thereby conferring a protective effect in patients with purulent myocarditis.31 Wang et al.’s study further corroborated that inhibition of the MAPK/ERK signaling pathway suppresses keratinocyte proliferation in an inflammatory cell line model stimulated by cytokine mixtures, thereby attenuating inflammatory progression.32 Furthermore, activation of the ERK signaling pathway potentially triggers the onset of prostatitis and facilitates its progression. Accumulating evidence indicates that inflammatory responses are a pivotal factor in the pathogenesis of CP33 and play a crucial role in the pathological progression of CP. Hua et al., utilizing an experimental autoimmune prostatitis mouse model, demonstrated that C‒X‒C motif chemokine 10 (CXCL10) activates ERK and MAPK signaling pathways by binding to its receptor CXCR3, thereby inducing the accumulation of inflammatory cells and secretion of inflammatory mediators, resulting in prostate inflammatory infiltration and pain symptoms.34 Recent studies have established that certain drugs effectively ameliorate symptoms of CP by inhibiting the ERK signaling pathway, thereby reducing oxidative stress and inflammatory responses.33 Additionally, activation of the ERK signaling pathway is implicated in regulating cell growth and differentiation processes, and its aberrant activation plays a pivotal role in tumor development and progression. A substantial body of literature has demonstrated that aberrant activation of MAPK signaling frequently induces human cancers or developmental disorders.35 Research has revealed that over 85% of cancers exhibit hyperactive MAPK signaling,36 which substantially accelerates disease progression. Consequently, the development of anti-tumor drugs targeting various protein members of the RAS‒RAF‒MEK‒ERK (MAPK) signaling pathway has attracted considerable attention.37 In recent decades, various small molecule compounds targeting ERK1/2 have been developed and implemented in preclinical and clinical cancer research.38 In conclusion, targeted inhibition of the ERK signaling pathway may offer novel therapeutic strategies for preventing and treating ICI-related prostatitis as well as suppressing tumor growth.
In addition to the ERK pathway, the IL-6 signaling pathway in murine prostate tissue demonstrates significant activation following ICI treatment, indicating that the IL-6 signaling pathway may play a crucial role in the pathogenesis of ICI-induced prostatitis. IL-6, a key pro-inflammatory cytokine, plays a central role in the pathophysiological processes of various inflammatory diseases. Elevated urinary IL-6 levels in patients with acute kidney injury or mesangial proliferative glomerulonephritis, for example, are frequently associated with poor clinical prognosis.39 Furthermore, studies utilizing animal models have demonstrated that IL-6 can induce renal inflammatory responses, exacerbate tissue damage, and result in increased proteinuria.40 In the pathogenesis of inflammatory or degenerative arthritis, IL-6 exhibits pleiotropic effects, including the promotion of synovial hyperplasia, maintenance of joint inflammatory states, and acceleration of degenerative damage to cartilage and bone.41 Clinical correlation studies and experimental data collectively substantiate that IL-6 trans-signaling plays a pivotal role in modulating the extent of leukocyte infiltration and the severity of joint destruction.42 Consequently, IL-6 trans-signaling is postulated to be a key mechanism through which IL-6 mediates localized pathological damage. Recent studies have demonstrated that selective targeting of the IL-6 trans-signaling pathway in animal models of inflammatory diseases frequently results in significant improvement of disease prognosis.43 Accumulating evidence indicates that the IL-6 signaling pathway may orchestrate inflammatory processes through multiple mechanisms, encompassing the recruitment of inflammatory cells and the promotion of cytokine release, among others. IL-6 elicits profound effects on diverse cell types, including stromal cells, tissue-resident monocytes, and activated inflammatory leukocytes. This pleiotropic effect enables IL-6 to play a pivotal role in the initiation, perpetuation, and resolution of both local and systemic inflammatory responses that contribute to tissue damage. At sites of tissue injury, members of the IL-6 cytokine family function as key mediators, driving the cascade of inflammation-induced tissue damage. Studies have demonstrated that IL-6 plays a critical role in modulating the recruitment of inflammatory cells.44 Concurrently, IL-6 stimulates hepatic production and release of acute-phase proteins, including C-reactive protein, serum amyloid P component, serum amyloid A, fibrinogen, and ferritin, into the bloodstream.45 A substantial body of literature has reported that elevated IL-6 levels are strongly correlated with various chronic conditions, including inflammatory prostatitis.46 Chen et al. demonstrated that IL-6 upregulation accelerates macrophage-induced prostate cancer progression.47 Currently, IL-6 is recognized as one of the most reliable surrogate markers for chronic inflammation associated with prostate cancer.48 Nevertheless, comprehensive investigations into the specific mechanisms by which IL-6 contributes to the initiation and progression of prostatitis remain limited.
Recent studies have demonstrated that targeted therapies against IL-6 or its receptor, particularly tocilizumab, have been successful in treating various immune disorders.49 Targeting the IL-6 signaling pathway has demonstrated significant clinical translational potential in treating irAEs, particularly prostatitis, especially in patients with elevated IL-6 levels. Therapeutic interventions targeting the ERK pathway, specifically ERK inhibitors, have demonstrated efficacy in various cancer treatments.38 Consequently, targeting the ERK pathway represents an emerging therapeutic strategy for managing ICI-associated irAEs, particularly prostatitis. This targeted approach can modulate the intensity of the immune response, thus attenuating inflammatory processes and reducing the incidence of irAEs, particularly prostatitis. Continuous monitoring of the IL-6 and ERK signaling pathway activation status enables the implementation of more precise therapeutic strategies. In patients with elevated activation of IL-6 or ERK signaling pathways, early implementation of targeted therapy can effectively prevent or reduce irAEs, thereby improving both treatment tolerance and quality of life. Future clinical trials will be essential to verify the efficacy of targeting IL-6 or ERK signaling pathways in managing ICI-induced irAEs, particularly prostatitis. These findings will establish new strategies for irAE management in ICI therapy and facilitate the development of individualized treatment protocols.
This study has several limitations that necessitate further exploration and refinement in subsequent research. First, the FAERS database, as a spontaneous reporting system, is inherently susceptible to systematic biases, including underreporting and misreporting. In practice, cases may be either unreported or incompletely documented within the FAERS system. Notably, minor adverse events often go undocumented, potentially introducing reporting bias. The heterogeneity in reporting quality among different sources (healthcare professionals, patients, and pharmaceutical manufacturers) can result in significant data inconsistencies. Individual case reports frequently contain incomplete or imprecise information, which can compromise the validity of subsequent analyses. Furthermore, limitations in the FAERS database regarding overall survival, disease-free survival, and temporal patterns and severity of ADRs substantially restricted our comprehensive analysis of clinical outcomes in patients receiving ICIs. Second, while animal models provide valuable evidence for mechanistic studies, they may not fully recapitulate human pathological processes; consequently, relevant mechanisms require validation in human subjects. In this study, we utilized mouse models, focusing solely on comparing the differences in signaling pathway activation levels in prostate tissues between the ICI-treated group and the untreated control group. The adverse events associated with ICI-induced prostatitis observed in our study require clinical validation, and current literature in this field remains limited. Given the preliminary and exploratory nature of this study, large-scale prospective cohort studies are essential to further validate our findings. Such studies would enable a more accurate assessment of the true incidence of ICI-associated prostatitis, a comprehensive elucidation of its potential biological mechanisms and risk factors, thus providing more robust evidence for clinical risk management.
5 CONCLUSIONS
This study systematically investigated the incidence, risk factors, and potential biological mechanisms of ICI-related prostatitis through a comprehensive analysis of the FAERS database and the establishment of preclinical mouse models. The results demonstrated that ICI therapy significantly increased the risk of prostatitis, with a statistically significant difference observed between treatment and control groups. Notably, the occurrence of prostatitis did not show a significant correlation with patient age; however, it may potentially impact patient prognosis. Further investigation using preclinical mouse models revealed that ICI therapy may induce prostate inflammation by activating the IL-6 and ERK signaling pathways, as evidenced by increased expression of inflammatory markers and pathway-specific proteins. Based on these findings, we hypothesize that inhibiting the IL-6 and ERK signaling pathways may alleviate symptoms of ICI-related prostatitis, potentially offering a novel therapeutic approach. These findings not only significantly deepen our understanding of ICI-related prostatitis but also provide an important theoretical basis for risk assessment and management in clinical practice, potentially informing future guidelines for patient care. Looking ahead, targeted therapies against IL-6 and ERK pathways may emerge as novel strategies for preventing and treating ICI-related prostatitis, potentially improving patients' quality of life and further optimizing ICI treatment regimens. However, further clinical studies are needed to validate these findings and assess the efficacy and safety of such interventions. The results of this study highlight the necessity of closely monitoring male reproductive system adverse events during ICI therapy, thus providing innovative insights for developing personalized cancer immunotherapy strategies and potentially improving patient outcomes.
AUTHOR CONTRIBUTIONS
Writing—original draft: Peng Luo, Ying Liu, Lan Xu, Quan Cheng, Anqi Lin, Xiao Fang, and Aimin Jiang. Conceptualization: Quan Cheng, Anqi Lin, Xiao Fang, and Aimin Jiang. Investigation: Peng Luo, Ying Liu, and Lan Xu. Writing—review and editing: Peng Luo, Ying Liu, Lan Xu, Zaoqu Liu, Linhui Wang, Hank Z.H. Wong, Quan Cheng, Anqi Lin, Xiao Fang, and Aimin Jiang. Visualization: Peng Luo, Ying Liu, Lan Xu, and Anqi Lin. All authors have read and agreed to the published version of the manuscript.
ACKNOWLEDGMENTS
This work was supported by grants from the Shanghai Municipal Health Commission Science Research Project (20214Y0320) and the Naval Medical University Basic Medical Science Research Program (2022MS028).
CONFLICT OF INTEREST STATEMENT
The authors declare they have no conflict of interest.