The effectiveness and safety of azvudine treatment in COVID-19 patients with kidney disease based on a multicenter retrospective cohort study
Bo Yu, Mengzhao Yang, and Jia Yu contributed equally to this work.
Abstract
Kidney disease has been the main risk factor of poor prognosis for COVID-19 patients. The effectiveness and safety of azvudine treatment in COVID-19 patients with kidney disease have not been reported. Herein, we conducted a nine-center and retrospective cohort study in China (ClinicalTrials: NCT06349655) that enrolled 32,864 hospitalized COVID-19 patients, in which 4192 patients were pre-existed with kidney disease. After exclusions and propensity score matching, a total of 831 kidney disease patients treated with azvudine and 831 kidney disease patients treated without any anti-viral treatment (normal group) were selected. Based on Kaplan–Meier and Cox regression analysis, we found that azvudine administration had significantly decreased risks of all-cause death (p < 0.0001) and composite disease progression (p = 0.012) as compared to the normal group. Multivariate Cox proportional hazards regression analysis demonstrated that the hazard ratio of all-cause death was 0.64 (95% CI: 0.503–0.826, p < 0.001) and the hazard ratio of composite disease progression was 0.81 (95% CI: 0.658–1.004, p = 0.05). The subgroup analysis of different characteristics indicated no significant influence of single factor in both all-cause mortality and composite disease progression. Five sensitivity analyses were employed to verify the robustness of our results. Safety analysis based on adverse event rate demonstrated an increased rate of hypertriglyceridemia after azvudine administration. In conclusion, we are the first to report the effectiveness and safety of azvudine treatment in COVID-19 patients with kidney disease and demonstrate that azvudine could reduce the risk of all-cause death without significant adverse events based on a large-scale, multicenter, retrospective cohort study.
1 INTRODUCTION
Respiratory viral infections are one of the most common diseases globally, and emerging viruses such as Wuhan pneumonia caused by a novel coronavirus pose a major threat to global public health.[1] As of February 4, 2024, it continues to strain healthcare systems worldwide, with over 774 million reported infections and 7 million deaths.[2] Although vaccination mitigates the impact of COVID-19 in vulnerable groups, it has not been effective in stopping infections by the COVID-19 variant, which displays significant immune evasion.[3] Moreover, policy changes have led to the discontinuation of isolation protocols for COVID-19 patients, further elevating the pressure on healthcare facilities. Consequently, the need for selecting effective treatments has become increasingly critical. Although the prevalence of kidney disease and the frequency of complications are higher in COVID-19 patients, a systematic evaluation and meta-analysis found a combined rate of acute kidney injury (AKI) of 28% in 30,657 hospitalized COVID-19 patients.[4] Another study showed that 5.1% of 701 hospitalized COVID-19 patients developed AKI, while 43.9% had proteinuria on admission. This shows that kidney injury is a relatively common phenomenon in COVID-19 patients, and therefore there is an urgent need for a therapeutic regimen for COVID-19 patients with kidney injury.[5] At the same time, it has been shown that patients with AKI may develop pulmonary edema and require mechanical ventilation, mainly due to the inflammatory response and increased vascular permeability caused by increased systemic immune mediators.[6] This can also further aggravate the condition caused by COVID-19.
Several drugs have been developed on the market for the treatment of COVID-19, including paxlovid, molnupiravir, azvudine, and fluvoxamine.[7, 8] Among them, azvudine is the first independently developed oral small molecule COVID-19 therapeutic drug in China, which received conditional approval in July 2022 for the treatment of adult patients with COVID-19 in China.[9] Our previous controlled clinical trial demonstrated that patients in the azvudine group had a significantly shorter time to first nucleic acid negative than the control group, indicating the potential efficacy and good tolerability of azvudine in the treatment of mild and common COVID-19 patients.[10] Importantly, another study showed that azvudine was able to reduce the time to nucleic acid negative, viral load, and time to clinical improvement, demonstrating a favorable safety and tolerability profile.[8] However, definitive reports of the therapeutic efficacy of azvudine in COVID-19 patients with kidney disease do not exist at this time.
Therefore, we collected 32,864 samples and conducted this large-scale, multicenter, retrospective cohort study to investigate the efficacy and safety of oral azvudine for the treatment of hospitalized COVID-19 patients with kidney disease. In addition, we performed several sensitivity analyses to validate our findings.
2 METHODS
2.1 Data sources
We collected data on hospitalized patients diagnosed with SARS-CoV-2 (COVID-19) infection by reverse transcription polymerase chain reaction (RT-PCR) from December 5, 2022, to January 31, 2023, at nine hospitals in Henan Province, China, including the First Affiliated Hospital of Zhengzhou University, Henan Provincial Chest Hospital, Henan Infectious Disease Hospital, Luoyang Central Hospital, Nanyang Central Hospital, the Fifth People’s Hospital of Anyang, Shangqiu Municipal Hospital, Guangshan County People’s Hospital, and Fengqiu County People’s Hospital. We retrieved the electronic medical records of these patients, including demographic characteristics, date of hospitalization, admission time, registered deaths, diagnoses, prescription and dispensing records, procedures, imaging data, and laboratory tests.
2.2 Inclusion criteria for the study population
Inclusion criteria were as follows: (1) patients aged 18 years and older; (2) SARS-CoV-2 RT-PCR positive; (3) receiving standard treatment or standard treatment plus azvudine; and (4) patients suffering from kidney disease. Exclusion criteria were as follows: (1) patients were treated with other antiviral drugs in addition to azvudine treatment and (2) patients themselves had some allergic reaction to azvudine. Patients were diagnosed and treated according to the “COVID-19 Diagnosis and Treatment Program (Trial Version 9 or 10)” issued by the National Health Commission of the People's Republic of China.[11, 12]
2.3 Procedures
The selected participants were assigned to either the azvudine group (5 mg once daily for a maximum of 14 days) or the normal group (received only standard treatment) based on their drug prescription and dispensing records. The starting point for the study was considered as the date of confirmed SARS-CoV-2 infection. Matching of the normal and azvudine groups was done through propensity scores (PSM) in a 1:1 ratio. Patients were monitored from the initial date until the occurrence of the outcome event or up to 30 days. Approval for this study was obtained from the research ethics committee at the First Affiliated Hospital of Zhengzhou University with the approval number 2023-KY-0865-001. The study protocol followed the STROBE guidelines and the ethical principles established in the 1975 Declaration of Helsinki. Written informed consent was obtained from all participants involved in the study. Our clinical trial registration number is NCT06349655 on ClinicalTrials.
2.4 Statistical analysis
The research utilized R v. 4.0.3 for data analysis, where statistical significance was defined by a two-tailed p-value below 0.05. Mean and standard deviation were reported for normally distributed continuous variables, while median and interquartile range were utilized for non-normally distributed variables. Group differences in continuous variables were evaluated through appropriate methods such as independent t-tests or Mann–Whitney U tests. Categorical variables were presented as frequencies and percentages, with group differences analyzed using the chi-square test. Multiple imputations were implemented to account for missing values. To mitigate the influence of confounding variables on intervention evaluation, a 1:1 greedy matching approach was employed to match baseline characteristics (e.g., age, gender, severity, BMI, concomitant systemic steroid, time from diagnosis to treatment initiation, and comorbidities) between the normal and azvudine groups using logistic regression. The equality of baseline characteristics between the two groups was assessed based on the p-value, with p > 0.05 indicating satisfactory balance.
The Kaplan–Meier method was utilized to generate cumulative event curves, while the log-rank test was employed to assess the difference in survival between groups. Cox proportional hazards regression models were developed to determine hazard ratios (HRs) and their corresponding 95% confidence intervals (CIs) for both primary and secondary outcomes, adjusting for baseline covariates. The assumption of proportional hazards was verified using Schoenfeld residuals, and multicollinearity was tested using the variance inflation factor (VIF), with a VIF value exceeding 5 indicating multicollinearity. Subgroup analysis was carried out, with stratification based on factors such as gender, age, severity, and comorbidities. To ensure the reliability of the study findings, sensitivity analyses were conducted. Initially, missing values were imputed using the mean, and a logistic regression model was used to perform a 1:1 greedy match to compare results. A probabilistic model was then employed for a 1:1 greedy match. Additionally, individuals discharged from the hospital on the first day after admission were excluded to account for the time needed for the drug to take effect and reach peak blood concentration, thereby narrowing the study population.
2.5 Definition of covariates
Demographic information such as age, gender, and body mass index (BMI) was gathered from the patients upon admission. Patients were classified as having a “mild”, “moderate”, or “severe” condition based on the COVID-19 diagnosis and treatment guidelines. The use of hormone therapy within 24 h of admission was also noted. Furthermore, the timing of azvudine administration was recorded as either “>5 days” or “0–5 days” from the initial diagnosis. Laboratory results at the time of diagnosis included neutrophil (Neut), lymphocyte (Lymph), glucose (Glu), high-density lipoprotein (HDL), low-density lipoprotein (LDL), alanine aminotransferase (ALT), aspartate aminotransferase (AST), creatinine (CREA), glomerular filtration rate (GFR), C-reactive protein (CRP), procalcitonin (PCT), prothrombin time (PT), activated partial thromboplastin time (APTT), cholesterol (CH), triglyceride (TG), alkaline phosphatase (ALP), gamma-glutamyl transpeptidase (GGT), albumin (ALB), and total bilirubin (TBIL) whose levels were also documented. Additionally, information on the presence of diabetes, hypertension, liver diseases, cardio-cerebral diseases, and primary malignant tumors was extracted from electronic records.
2.6 Outcomes
The main result of this research focuses on total mortality, with a secondary emphasis on a combined progression of illness. Mortality data were obtained from digital patient records. Progression of illness encompasses mortality, advancement from mild or moderate to severe or critical illness, as well as mortality or progression to critical disease in severe cases. Severe illness is characterized by a respiratory rate of 30 times per minute or higher, oxygen saturation at rest of 93% or less, a PaO2/FiO2 ratio below 300 mmHg, or lung lesions progressing by over 50% within 24–48 h. Critical disease involves the need for mechanical ventilation, shock, or intensive care unit monitoring. To assess safety, overall adverse events (AEs) and AEs graded as severe (grade ≥ 3) were analyzed. AEs were classified according to the guidelines outlined in the Common Terminology Criteria for Adverse Events, Version 5.0 (CTCAE 5.0).[13] The primary AEs monitored in this study were abnormal laboratory findings. The initiation point for data collection was established post-drug administration, and the termination point for follow-up was defined as five half-lives following the final dose. In cases where the severity of an AE fluctuated, the highest grade observed was recorded.
3 RESULTS
3.1 Baseline characteristics
Data were collected on a total of 32,864 confirmed SRAS-CoV-2 infection patients admitted to nine Hospitals in Henan Province (Figure 1). According to the inclusion and exclusion criteria, 4192 kidney patients were selected. A total of 831 patients taking azvudine and 3361 patients only take general treatment were included in this group. Subsequently, we used a 1:1 PSM to control for confounders, and finally included 831 patients in the azvudine group and 831 patients in the normal group.
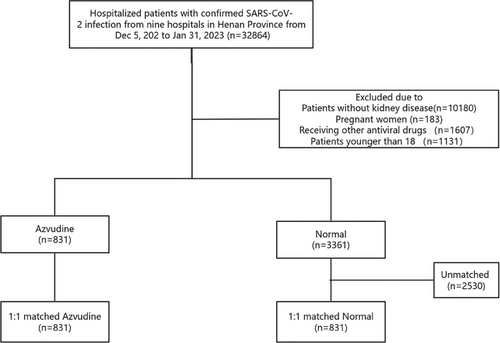
Before matching, the mean age was 64.43 years in the normal group and 67.33 years in the azvudine group (p < 0.001, standardized mean differences (SMD) < 0.001) (Table 1). There were many imbalances in variables between the two groups, such as gender, severity at admission, concomitant systemic steroid, liver diseases, cardio-cerebral diseases, primary malignant tumor, neutrophil, lymphocyte, glucose, prothrombin time, activated partial thromboplastin time, and alkaline phosphatase. After matching, all variables were well controlled between the two groups, and there were no significant differences (Figure S1).
| Before matching | After 1:1 matching | |||||
|---|---|---|---|---|---|---|
| Characteristics | Normal (n = 3361) | Azvudine (n = 831) | p value | Normal (n = 831) | Azvudine (n = 831) | p value |
| Age, mean (SD), year | 64.43(15.52) | 67.33(15.28) | <0.001 | 66.65 (15.42) | 67.33 (15.28) | 0.368 |
| Gender, n (%) | 0.003 | 0.874 | ||||
| Male | 2099(62.5) | 565 (68.0) | 569 (68.5) | 565 (68.0) | ||
| Female | 1262 (37.5) | 266 (32.0) | 262 (31.5) | 266 (32.0) | ||
| BMI, mean (SD), kg/m2 | 23.72 (3.85) | 24.07 (4.05) | 0.021 | 24.17 (4.07) | 24.07 (4.05) | 0.608 |
| Severity at admission, n (%) | <0.001 | 0.811 | ||||
| Mild | 291 (8.7) | 62 (7.5) | 64 (7.7) | 62 (7.5) | ||
| Moderate | 2428 (72.2) | 487 (58.6) | 474 (57.0) | 487 (58.6) | ||
| Severe | 642 (19.1) | 282 (33.9) | 293 (35.3) | 282 (33.9) | ||
| Vaccinationdoses (%) | 0.008 | 0.932 | ||||
| 0 | 1186 (35.3) | 336 (40.4) | 334 (40.2) | 336 (40.4) | ||
| 1 | 186 (5.5) | 58 (7.0) | 64 (7.7) | 58 (7.0) | ||
| 2 | 562 (16.7) | 110 (13.2) | 116 (14.0) | 110 (13.2) | ||
| 3 | 1393 (41.4) | 319 (38.4) | 311 (37.4) | 319 (38.4) | ||
| 4 | 34 (1.0) | 8 (1.0) | 6 (0.7) | 8 (1.0) | ||
| Antibiotics (%) | <0.001 | 0.766 | ||||
| No | 2018 (60.0) | 357 (43.0) | 364 (43.8) | 357 (43.0) | ||
| Yes | 1343 (40.0) | 474 (57.0) | 467 (56.2) | 474 (57.0) | ||
| Concomitant systemic steroid, n (%) | <0.001 | 0.921 | ||||
| No | 2531 (75.3) | 484 (58.2) | 504 (60.6) | 484 (58.2) | ||
| Yes | 830 (24.7) | 347 (41.8) | 327 (39.4) | 347 (41.8) | ||
| Comorbidities, n (%) | ||||||
| Diabetes | 830 (24.7) | 347 (41.8) | <0.001 | 327 (39.4) | 347 (41.8) | 0.343 |
| Hypertension | 1343 (40.0) | 474 (57.0) | <0.001 | 467 (56.2) | 474 (57.0) | 0.766 |
| Hepatopathy (%) | 1111 (33.1) | 277 (33.3) | 0.911 | 278 (33.5) | 277 (33.3) | 1 |
| Cardio-cerebral diseases | 1703 (50.7) | 493 (59.3) | <0.001 | 491 (59.1) | 493 (59.3) | 0.96 |
| Primary malignant tumor | 615 (18.3) | 127 (15.3) | 0.047 | 122 (14.7) | 127 (15.3) | 0.783 |
| Chronic respiratory diseases (%) | 460 (13.7) | 122 (14.7) | 0.492 | 34 (4.1) | 35 (4.2) | 1 |
| Auto immune diseases (%) | 223 (6.6) | 35 (4.2) | 0.012 | 34 (4.1) | 35 (4.2) | 1 |
| Laboratory parameters, mean (SD) | ||||||
| Neutrophil, ×109/L | 6.30 (4.97) | 6.36 (4.34) | 0.766 | 6.54 (4.51) | 6.36 (4.34) | 0.386 |
| Lymphocyte, ×109/L | 1.06 (0.83) | 0.85 (0.61) | <0.001 | 0.85 (0.54) | 0.85 (0.61) | 0.801 |
| Glucose, mmol/L | 7.79 (5.17) | 9.07 (5.83) | <0.001 | 9.05 (6.61) | 9.07 (5.83) | 0.94 |
| High-density lipoprotein, mmol/L | 1.02 (0.92) | 0.98 (0.34) | 0.314 | 0.97 (0.34) | 0.98 (0.34) | 0.303 |
| Low-density lipoprotein, mmol/L | 2.27 (1.29) | 2.11 (1.01) | 0.001 | 2.11 (1.04) | 2.11 (1.01) | 0.997 |
| Alanine aminotransferase, IU/L | 44.52 (253.67) | 33.68 (70.22) | 0.222 | 30.02 (49.55) | 33.68 (70.22) | 0.22 |
| Aspartate aminotransferase, IU/L | 68.49 (438.74) | 44.13 (97.90) | 0.112 | 41.23 (70.66) | 44.13 (97.90) | 0.489 |
| Creatine, µmol/L | 330.03 (610.63) | 287.45 (383.16) | 0.055 | 298.72 (334.78) | 287.45 (383.16) | 0.523 |
| Glomerular filtration rate, mL/min | 72.84 (136.29) | 63.97 (106.59) | 0.08 | 66.86 (127.37) | 63.97 (106.59) | 0.616 |
| C-reactive protein, mg/L | 58.27 (72.90) | 69.84 (88.11) | <0.001 | 68.05 (80.65) | 69.84 (88.11) | 0.666 |
| Procalcitonin, ng/mL | 4.24 (16.92) | 2.51 (10.21) | 0.005 | 2.53 (8.64) | 2.51 (10.21) | 0.967 |
| Prothrombin time, s | 14.40 (9.13) | 17.96 (13.40) | <0.001 | 16.94 (12.26) | 17.96 (13.40) | 0.105 |
| Activated partial thromboplastin time, s | 30.72 (14.94) | 27.29 (15.08) | <0.001 | 27.86 (9.89) | 27.29 (15.08) | 0.359 |
| Cholesterol, mmol/L | 4.29 (4.65) | 3.90 (1.21) | 0.017 | 3.89 (1.40) | 3.90 (1.21) | 0.896 |
| Triglyceride, mmol/L | 2.03 (6.44) | 1.87 (3.70) | 0.493 | 1.94 (6.61) | 1.87 (3.70) | 0.796 |
| Alkaline phosphatase, IU/L | 100.32 (100.49) | 88.86 (64.00) | 0.002 | 91.52 (54.13) | 88.86 (64.00) | 0.361 |
| Gamma-glutamyl transpeptidase, IU/L | 59.47 (95.25) | 60.57 (105.92) | 0.772 | 57.15 (94.77) | 60.57 (105.92) | 0.488 |
| Albumin, g/L | 36.32 (47.30) | 37.56 (79.45) | 0.561 | 37.37 (64.00) | 37.56 (79.45) | 0.957 |
| Total bilirubin, µmol/L | 15.69 (40.78) | 10.91 (10.23) | 0.001 | 11.80 (14.16) | 10.91 (10.23) | 0.143 |
3.2 The primary and secondary outcome
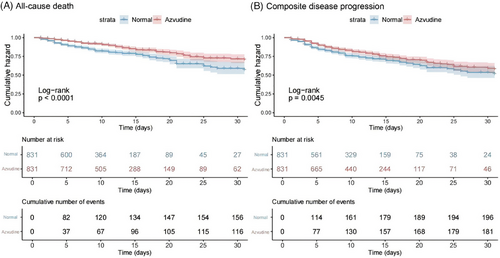
The primary outcome was all-cause death. During follow-up, there were 200 events in the azvudine group and 136 events in the normal group. Kaplan–Meier method showed that the normal group had a higher risk of all-cause death compared with the azvudine group (log-rank p < 0.0001) (Figure 2A). The secondary outcome was composite disease progression. During follow-up, a total of 190 events was occurred in the normal group and 182 events in the azvudine group. The results also showed that oral azvudine slowed the progression of the disease (p = 0.0045) (Figure 2B).
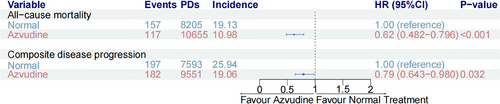
The crude incidence rate of all-cause death was 20.70 per 1000 person-days in the normal group versus 12.04 per 1000 person-days in the azvudine group (Figure 3). Furthermore, all baseline variables were further controlled by Cox multivariate regression model, and the results showed that the risk of all-cause death was decreased by 38% in the azvudine group compared with the normal group (HR: 0.62, 95% CI: 0.482–0.769, p < 0.001) (Figure 3). On the other hand, the crude incidence rate of composite disease progression was 25.94 per 1000 person-days in the normal group versus 19.06 per 1000 person-days in the azvudine group. Cox multivariate regression model showed the risk was lower in the azvudine group compared to the normal groups in the risk of composite disease progression (HR: 0.79, 95% CI: 0.643−0.980, p = 0.032) (Figure 3).
We further stratified patients according to gender, severity, concomitant systemic steroid, age, time from diagnosis to treatment exposure, diabetes, hypertension, liver disease, cardio-cerebral diseases, and primary malignant tumor for subgroup analysis (Table 2). We found that in reducing the risk of composite diseases, the effect of azvudine was slightly weaker in patients with hypertension, and there was no impact on the risk of all-cause mortality. None of the other individual factors had a significant impact on the risk of all-cause mortality or composite disease progression, indicating that our results are largely unaffected by the remaining factors.
| All-cause death | Composite disease progression | |||
|---|---|---|---|---|
| Characteristic | HR (95% CI) | p value for interaction | HR (95%CI) | p value for interaction |
| Gender | ||||
| Male | 0.57 (0.43−0.76) | 0.954 | 0.69 (0.55−0.88) | 0.274 |
| Female | 0.57 (0.36−0.91) | 0.89 (0.60−1.32) | ||
| Severity at admission | ||||
| Mild | 0.52 (0.14−1.94) | 0.855 | 0.33 (0.06−1.83) | 0.567 |
| Moderate | 0.53 (0.31−0.90) | 0.72 (0.44−1.18) | ||
| Severity | 0.60 (0.46−0.79) | 0.77 (0.62−0.96) | ||
| Vaccination doses | ||||
| 0 | 0.59 (0.41−0.84) | 0.99 | 0.71 (0.53−0.97) | 0.783 |
| 1 | 0.53 (0.25−1.14) | 1.02 (0.51−2.06) | ||
| 2 | 0.63 (0.33−1.22) | 0.81 (0.43−1.53) | ||
| 3 | 0.53 (0.34−0.82) | 0.70 (0.49−0.98) | ||
| 4 | 0.27 (0.02−3.06) | 0.22 (0.02−2.14) | ||
| Antibiotics | ||||
| No | 0.67 (0.45−1.01) | 0.323 | 0.79 (0.57−1.11) | 0.618 |
| Yes | 0.53 (0.39−0.71) | 0.72 (0.56−0.93) | ||
| Concomitant systemic steroid, n (%) | ||||
| No | 0.69 (0.51−0.93) | 0.055 | 0.69 (0.53−0.89) | 0.577 |
| Yes | 0.43 (0.29−0.64) | 0.84 (0.61−1.14) | ||
| Age | ||||
| ≤60 Years | 0.85 (0.51−1.40) | 0.057 | 0.76 (0.49−1.19) | 0.914 |
| >60 Years | 0.51 (0.39−0.67) | 0.74 (0.59−0.93) | ||
| Diabetes, n (%) | ||||
| No | 0.50 (0.37−0.68) | 0.126 | 0.68 (0.53−0.87) | 0.14 |
| Yes | 0.73 (0.49−1.10) | 0.92 (0.64−1.33) | ||
| Hypertension, n (%) | ||||
| No | 0.46 (0.33−0.65) | 0.08 | 0.58 (0.44−0.78) | 0.016 |
| Yes | 0.70 (0.50−0.98) | 0.94 (0.71−1.25) | ||
| Liver diseases, n (%) | ||||
| No | 0.57 (0.43−0.75) | 0.995 | 0.75 (0.60−0.95) | 0.791 |
| Yes | 0.57 (0.36−0.89) | 0.71 (0.46−1.08) | ||
| Cardio-cerebral diseases, n (%) | ||||
| No | 0.52 (0.37−0.74) | 0.344 | 0.76 (0.57−1.00) | 0.954 |
| Yes | 0.66 (0.48−0.92) | 0.76 (0.56−1.02) | ||
| Primary malignant tumor, n (%) | ||||
| No | 0.58 (0.45−0.75) | 0.681 | 0.76 (0.62−0.94) | 0.573 |
| Yes | 0.33 (0.12−0.91) | 0.43 (0.17−1.09) | ||
| Chronic respiratory diseases, n (%) | ||||
| No | 0.62 (0.48−0.80) | 0.136 | 0.78 (0.62−0.97) | 0.328 |
| Yes | 0.37 (0.19−0.75) | 0.62 (0.38−1.02) | ||
| Autoimmune diseases, n (%) | ||||
| No | 0.58 (0.46−0.74) | 0.567 | 0.75 (0.61−0.92) | 0.893 |
| Yes | 0.40 (0.04−4.45) | 0.81 (0.18−3.72) | ||
- Abbreviations: 95% CI, 95% confidence interval; HR, hazard ratio.
3.3 Sensitivity analysis
Five sensitivity analyses were conducted to test the robustness of our results. First, we used a Poisson regression model for propensity score matching and the baseline characteristics were well balanced between two groups (p > 0.05, SMD < 0.1). The baseline characteristics are shown in Table S1. The two sets of data were matched simultaneously, and the matched data are shown in Figure S2. The results showed that compared with the azvudine group, the normal group had a higher risk of all-cause death (log-rank p < 0.0001, Figure S3) and composite disease progression (log-rank p = 0.0013, Figure S4) through Kaplan–Meier curve. Cox regression analysis showed patients taking oral azvudine had a lower all-cause mortality rate compared to the Normal treatment (HR: 0.59, 95% CI: 0.457−0.754, p < 0.001, Figure S5). Cox regression analysis also showed a similarly superior performance with azvudine in the risk of composite disease progression between the two groups (HR: 0.77, 95% CI: 0.678–1.034, p = 0.018, Figure S5).
Second, we used the imputation of missing data mean to perform propensity score matching. The data situation after interpolation is shown in Figure S6, which demonstrates that all missing data are filled in.The baseline characteristics are shown in Table S2. The two sets of data were matched simultaneously, and the matched data are shown in Figure S7. The risk of all-cause death was significant higher in the normal group compared with the azvudine group through the Kaplan–Meier curve (log-rank p < 0.0001, Figure S8) and Cox regression (HR: 0.62, 95% CI: 0.479–0.793, p < 0.001, Figure S10). Kaplan–Meier curve (log-rank p = 0.0086, Figure S9) and Cox regression (HR: 0.78, 95% CI: 0.632–0.968, p = 0.01, Figure S10) also showed that azvudine can slow the progression of disease.
Third, the results remained robust when repeated analysis was conducted by excluding the population who was discharged from the hospital on the first day after admission (Table S3, Figure S11). Patients with azvudine administration was associated with a lower all-cause death risk than normal administration through Kaplan–Meier analysis (p < 0.0001) (Figure S12) and Cox regression analysis (p < 0.001, HR: 0.62, 95% CI: 0.482−0.808) (Figure S14). Moreover, patients with azvudine administration showed a lower composite disease progression risk compared with Normal treatment (Kaplan–Meier analysis with p = 0.02, Figure S13), as well as showing the same trend in the risk of compound disease progression by Cox regression analysis (p = 0.005, HR: 0.73, 95% CI: 0.590–0.913) (Figure S14).
Fourth, in order to avoid having patients who were not capable of taking the medication because of the rapid progression of their disease, we narrowed the sample and validated it only for those patients who were clearly capable of taking the medication. The two sets of data were matched simultaneously, and the matched data are shown in Figure S15. Patients with azvudine administration was associated with a lower all-cause death risk than normal administration through Kaplan–Meier analysis (p = 0.0016) (Figure S16) and Cox regression analysis (p = 0.038, HR: 0.76, 95% CI: 0.588−0.986) (Figure S18). However, patients with azvudine administration had no significant difference in the risk of composite disease progression versus normal administration through Kaplan–Meier analysis (p = 0.074, Figure S17) and Cox regression analysis (p = 0.928, HR: 0.99, 95% CI: 0.893–1.236) (Figure S18). The results show that azvudine continues to have significant efficacy in reducing all-cause mortality.
Lastly, to eliminate potential selection bias in the matching process, we conducted an inverse-probability-of-treatment weighted to control confounding factors. Kaplan–Meier curves demonstrated that compared to the normal group, administration of azvudine significantly reduced the all-cause death risk (p < 0.001, Figure S19) and the risk of composite disease progression (p < 0.001, Figure S20) in patients. Cox analysis results showed that compared to the normal group, administration of azvudine significantly reduced the all-cause mortality in patients (p = 0.004, HR: 0.69, 95% CI: 0.535–0.892, Figure S21). However, there was no significant difference in composite disease progression (p = 0.242, HR: 0.87, 95% CI: 0.699–1.905, Figure S21) between the two groups.
3.4 Safety analysis
We assessed and compared the safety of azvudine and normal in nephrotic patients with COVID-19 through collecting AE in both groups (Table 3). In the safety analysis, there was no statistically significant difference in the overall incidence of AEs between the normal group and the azvudine group in most subgroups (p > 0.05). However, the incidence of AEs in the azvudine group was significantly higher than that in the normal group in the following AEs: decreased lymphocyte count (31% vs. 40%, p < 0.001), elevated alanine aminotransferase (17% vs. 24%, p = 0.03), and elevated alkaline phosphatase (7.2% vs. 12%, p = 0.034). The incidence of AEs in the azvudine group was significantly lower than that in the normal group in terms of elevated creatinine levels (24% vs. 19%, p = 0.046) and hypophosphatemia (19% vs. 12%, p = 0.041). Meanwhile, the incidence of severe AEs (grade ≥ 3) in the azvudine group was notably higher than that in the control group in certain aspects. For instance, significant differences were observed in cases of hypokalemia (5.5% vs. 9.8%, p = 0.007) and decreased lymphocyte count (19% vs. 27%, p < 0.001).
| Available dataa | All grades | Grade ≥ 3b | ||||||
|---|---|---|---|---|---|---|---|---|
| Adverse events (n, %) | Normal | Azvudine | Normal | Azvudine | p value | Normal | Azvudine | p value |
| Lymphocyte count decreased | 731 | 758 | 224 (31%) | 303 (40%) | <0.001 | 142 (19%) | 205 (27%) | <0.001 |
| Lymphocyte count increased | 731 | 758 | 6 (0.8%) | 14 (1.8%) | 0.085 | 0 (0%) | 1 (0.1%) | >0.9 |
| Neutrophil count increased | 291 | 395 | 21 (7.2%) | 22 (5.6%) | 0.4 | 5 (1.7%) | 5 (1.3%) | 0.8 |
| PLT count decreased | 399 | 498 | 79 (20%) | 83 (17%) | >0.9 | 35 (8.8%) | 36 (7.2%) | 0.7 |
| Anemia | 330 | 408 | 141 (43%) | 201 (49%) | 0.077 | 75 (23%) | 88 (22%) | 0.7 |
| Hypophosphatemia | 196 | 266 | 37 (19%) | 32 (12%) | 0.041 | 0 (0%) | 0 (0%) | |
| Hypokalemia | 532 | 551 | 98 (18%) | 126 (23%) | 0.71 | 29 (5.5%) | 54 (9.8%) | 0.007 |
| Hyperkalemia | 532 | 551 | 73 (14%) | 69 (13%) | 0.6 | 35 (6.6%) | 27 (4.9%) | 0.2 |
| ALT increased | 334 | 459 | 58 (17%) | 109 (24%) | 0.03 | 14 (4.2%) | 20 (4.4%) | >0.9 |
| AST increased | 367 | 477 | 86 (23%) | 114 (24%) | 0.9 | 16 (4.4%) | 23 (4.8%) | 0.8 |
| ALP increased | 334 | 442 | 24 (7.2%) | 52 (12%) | 0.034 | 0 (0%) | 0 (0%) | |
| GGT increased | 286 | 377 | 42 (15%) | 67 (18%) | 0.3 | 1 (0.3%) | 6 (1.6%) | 0.2 |
| Hyperuricemia | 323 | 437 | 44 (14%) | 63 (14%) | 0.8 | 0 (0%) | 0 (0%) | |
| CREA increased | 385 | 503 | 94 (24%) | 95 (19%) | 0.046 | 41 (11%) | 37 (7.4%) | 0.086 |
| Hypoglycemia | 102 | 123 | 11 (11%) | 23 (19%) | 0.1 | 1 (0.5%) | 0 (0%) | >0.9 |
| Hypercholesterolemia | 60 | 119 | 2 (3.3%) | 11 (9.2%) | 0.2 | 0 (0%) | 0 (0%) | |
| Hypertriglyceridemia | 50 | 92 | 9 (18%) | 26 (28%) | 0.2 | 1 (2.0%) | 2 (2.2%) | >0.9 |
- Abbreviations: Hb, hemoglobin; ALT, alanine aminotransferase; ALP, alkaline phosphatase; AST, aspartate aminotransferase; CH, cholesterol; CREA, creatinine; PLT, platelets; Glu, glucose; UA, uric acid; TG, triglyceride.
- a Number of people who completed the follow-up of data collection for this indicator.
- b Severity grades were defined according to the National Cancer Institute Common Terminology Criteria for Adverse Events (CTCAE), version 5.0.
3.5 Dynamic changes in kidney function
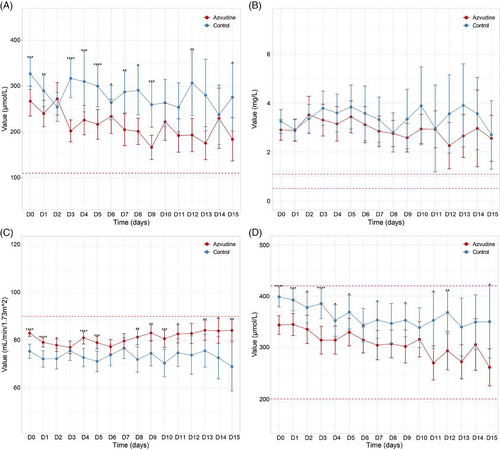
We further investigated the dynamic changes in kidney function in both normal and azvudine groups within 15 days of taking the drug. The results showed that creatinine and cystatin C were higher than the normal range in both groups, glomerular filtration rate was lower than the normal range, and uric acid index was within the normal range in both groups. Although creatinine and cystatin C were above the normal range in both groups, creatinine and cystatin levels in patients in the azvudine group were mostly lower than those in patients on normal treatment, and there were significant differences between the two groups at certain times of the day (Figure 4A,B). There was no significant difference in glomerular filtration rate between the two groups (Figure 4C), whereas uric acid levels in the azvudine group were generally lower than those of patients on normal treatment, with significant differences at certain times (Figure 4D).
4 DISCUSSION
After comparing a large amount of data and multiple samples, we can confirm that azvudine is superior to normal treatment in terms of efficacy, both in terms of reducing the risk of all-cause mortality and slowing down disease progression in patients with kidney disease COVID-19, while there is no significant difference in terms of safety from normal treatment. Subgroup analyses stratified by age, sex, concomitant systemic steroid use, diabetes mellitus, hypertension, hepatic disease, and cardioembolic disease yielded results that were largely consistent between the two groups, demonstrating that these additional conditions did not significantly interfere with our results. Our safety analysis indicates that patients taking azvudine may experience an increase in certain liver function indices compared to the normal group. This observation is somewhat related to the fact that azvudine is primarily metabolized by the liver. However, azvudine has been shown to decrease creatinine levels in the blood of patients, which can be beneficial for those with kidney disease. In COVID-19 patients with concomitant kidney disease, impaired renal function can lead to electrolyte disturbances, resulting in decreased blood potassium levels and consequently increasing the risk of hypokalemia.
In contrast to other studies of the same type, most previous studies have only controlled for the effects of confounding factors such as demographic characteristics, co-morbidities, medication use, and disease severity, and very few have controlled for the effects of laboratory test indices. Nevertheless, some studies have shown a correlation between white blood cell, lymphocyte, monocyte, and neutrophil counts at admission and mortality in COVID-19 patients.[14] In order to exclude the effects of these aspects, we controlled for factors related to demographic characteristics, laboratory test indicators, and drug use to further ensure the authenticity and reliability of the statistical results. In terms of data, in contrast to previous retrospective studies that used only one method of analysis or subgroup analysis, this study used five sensitivity analyses to repeatedly verify the robustness of the results. First, we used Poisson regression rather than logistic regression to control for confounders, which allowed for a more reasonable count of the number of disease occurrences.[15] Second, it has been shown that mean interpolation may have better performance when the mechanism of missing data does not depend on the outcome variable, and in order to re-verify the reliability of the results, we used mean interpolation instead of multiple interpolation for re-analysis.[16, 17] Third, considering that it takes time for the drug to work, we excluded some patients who experienced improvement, death or other factors resulting in discharge on the first day they received azvudine and reanalyzed them. Finally, we excluded patients who were prescribed but may not have taken their medication. All five sensitivity analysis methods mentioned above confirmed the results of this paper from different perspectives, again proving the reliability of our results.
The increase in the number of COVID-19 patients due to the current change in prevention policy has put our original treatment protocols to the test. Among the many COVID-19 patients, patients with kidney disease account for a significant portion of the population; for example, during the COVID-19 outbreak in Spain, 21% of the 1603 patients with an elevated serum creatinine level on admission to the hospital. Note that 43.5% of patients had chronic kidney disease.[18] These patients were also at higher risk than the general population. In a mortality analysis involving COVID-19 patients with chronic kidney disease, COVID-19 patients with chronic kidney disease had a significantly higher mortality rate than patients without kidney disease.[19] Moreover, the kidney is an important metabolic organ of the body, and numerous drugs are metabolized by the kidney and thus excreted from the body. Renal insufficiency not only affects the renal clearance of drugs, but also significantly alters the function of drug-metabolizing enzymes in both the liver and the kidney.[20] Therefore, for COVID-19 patients with kidney disease, the choice of drugs needs to be more careful. As the first independently developed oral small molecule COVID-19 therapeutic drug in China, azvudine has no serious nephrotoxicity reports, so it has a broad application prospect. Even some studies have shown that azvudine is not only effective in dialysis patients, but also has a good safety profile, which can significantly improve the condition and prognosis of patients.[21] Thus, it can be seen that azvudine is safe and effective for COVID-19 patients with kidney disease.
The kidney function tests in this paper also prove this phenomenon, in our report azvudine did not cause any significant change in glomerular filtration rate, which shows that azvudine does not further aggravate the burden on the kidneys. In addition, we detected lower levels of creatinine, cystatin C, and uric acid in patients taking azvudine, suggesting that kidney function was even improved in patients taking azvudine. This may be due to the fact that azvudine inhibits viral replication and reduces viral load, thereby reducing direct viral damage to the kidneys. Although the respiratory system is the main target of COVID-19, electron microscopy has shown that coronavirus particles are also present in renal tubular epithelium and podocytes, demonstrating that COVID-19 also directly attacks kidney cells.[22] Azvudine, a nucleoside antiviral drug, blocks the addition of nucleotides by mimicking natural nucleosides and being embedded during viral RNA synthesis, ultimately terminating RNA strand synthesis and viral replication.[23] This prevents the destruction of kidney cells by COVID-19 to some extent. Another possible reason is that azvudine was able to significantly reduce inflammatory markers (such as interleukin-6 and C-reactive protein), side-by-side demonstrating that azvudine was able to reduce the level of inflammatory response within the kidneys.[19] Although the inflammatory response is a protective response of the body, unresolved inflammation promotes progressive renal fibrosis, which exacerbates the burden on the kidneys, and azvudine was able to side-step kidney function by inhibiting the inflammatory response. Through the aforementioned methods, we speculate that azvudine may enhance renal function in patients with kidney disease, potentially mitigating long-term renal damage in individuals with chronic kidney disease and reducing the frequency of dialysis in patients with renal insufficiency.
In addition, azvudine has been shown to be superior to other COVID-19 therapeutics, as our previous studies have demonstrated that azvudine is better tolerated and has a lower rate of AEs than treatments such as interferon α, kaletra, ribavirin, chloroquine phosphate, and hydroxychloroquine sulfate.[9] Compared with the internationally accepted treatment paxlovid (nirmatrelvir/ritonavir) for COVID-19, azvudine also has a lower adverse rate. One study showing a drug-related AE rate of 7.16% in the azvudine group compared to 8.33% in the nirmatrelvir/ritonavir group.[24] Furthermore, a real-world retrospective cohort study showed that azvudine was superior to nirmatrelvir/ritonavir in reducing progression and lowering all-cause mortality, all of which demonstrates the superior efficacy of azvudine compared to other antiviral agents.[25] Particularly for patients with kidney disease, another paper discussed the pharmacokinetic and pharmacodynamic factors to be considered when using antiviral drugs in patients with kidney disease, pointed out the potential use of azvudine in patients with different stages of kidney function, and recommended azvudine as the first recommended therapeutic agent.[26]
There are some limitations to our study. First, we did not collect information on patients' vaccination, and the effect of patients' autoimmunization may have influenced the results. Considering that the vaccination rate in China is nearly 90% in adults, the lack of this information may have had little effect on the results.[27] Second, our follow-up date was only 30 days, and we lacked long-term follow-up data to further evaluate the efficacy. Third, with the ongoing evolution of the SARS-CoV-2 virus strain, further research is needed to determine the effectiveness of azvudine against different strains of the virus. Additionally, azvudine has the ability to improve kidney function. We need to further explore whether it has a broader application than just targeting COVID-19.
5 CONCLUSION
In conclusion, we are the first to report the effectiveness and safety of azvudine treatment in COVID-19 patients with kidney disease, and demonstrate that azvudine could reduce the risk of all-cause death without significant AEs based on a large-scale, multicenter, retrospective cohort study. Notably, azvudine not only reduced viral levels in patients, but also improved kidney function. Therefore, taking into consideration of the indications and improvement of patients' conditions, we recommend azvudine as the first choice of therapeutic agent for COVID-19 patients with kidney disease. And we hope that azvudine will be efficacious in all patients with kidney disease associated with respiratory disease, and that this paper will go some way to informing the treatment regimen for these patients.
AUTHOR CONTRIBUTIONS
Bo Yu, Mengzhao Yang, and Jia Yu contributed equally to this work. Quancheng Kan and Zhigang Ren conceived and designed the study. Zhigang Ren, Guotao Li, Shixi Zhang, Ling Wang, Hong Luo, Donghua Zhang, Silin Li, and Guowu Qian managed the patients. Mengzhao Yang, Danming Wang, Ming Cheng, and Ling Wang collected the data. Bo Yu, Mengzhao Yang, and Jia Yu analyzed the data. Bo Yu wrote the manuscript. All authors reviewed and approved the manuscript.
ACKNOWLEDGMENTS
This work was supported by the National Key Research and Development Program of China (2022YFC2303100 to Z.R.), Young and Middle-aged Academic Leaders of Henan Provincial Health Commission (HNSWJW-2022013 to Z.R.), and the Scientific Research and Innovation Team of The First Affiliated Hospital of Zhengzhou University (QNCXTD2023002 to Z.R.).
CONFLICT OF INTEREST STATEMENT
The authors declare no conflict of interest.
Open Research
DATA AVAILABILITY STATEMENT
The dataset used or analyzed during the current study are available from the corresponding author on reasonable request.