Copper-Catalyzed Oxidative Heterocyclization by Atmospheric Oxygen
Martin E. Bluhm Dr.
Institute for Technical Chemistry (ITC-CPV) Forschungszentrum Karlsruhe P.O. Box 3640, 76021 Karlsruhe (Germany) Fax: (+49) 7247-82-2244
Search for more papers by this authorMichael Ciesielski Dr.
Institute for Technical Chemistry (ITC-CPV) Forschungszentrum Karlsruhe P.O. Box 3640, 76021 Karlsruhe (Germany) Fax: (+49) 7247-82-2244
Search for more papers by this authorHelmar Görls Dr.
Department of Inorganic Chemistry University of Jena August-Bebel-Strasse 2, 07743 Jena (Germany)
Search for more papers by this authorManfred Döring Prof. Dr.
Institute for Technical Chemistry (ITC-CPV) Forschungszentrum Karlsruhe P.O. Box 3640, 76021 Karlsruhe (Germany) Fax: (+49) 7247-82-2244
Search for more papers by this authorMartin E. Bluhm Dr.
Institute for Technical Chemistry (ITC-CPV) Forschungszentrum Karlsruhe P.O. Box 3640, 76021 Karlsruhe (Germany) Fax: (+49) 7247-82-2244
Search for more papers by this authorMichael Ciesielski Dr.
Institute for Technical Chemistry (ITC-CPV) Forschungszentrum Karlsruhe P.O. Box 3640, 76021 Karlsruhe (Germany) Fax: (+49) 7247-82-2244
Search for more papers by this authorHelmar Görls Dr.
Department of Inorganic Chemistry University of Jena August-Bebel-Strasse 2, 07743 Jena (Germany)
Search for more papers by this authorManfred Döring Prof. Dr.
Institute for Technical Chemistry (ITC-CPV) Forschungszentrum Karlsruhe P.O. Box 3640, 76021 Karlsruhe (Germany) Fax: (+49) 7247-82-2244
Search for more papers by this authorGraphical Abstract
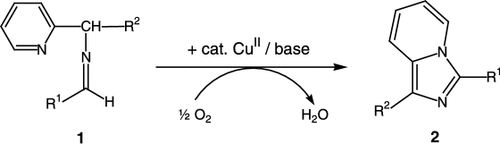
Starting from Schiff bases 1, a wide variety of heterobicycles, which can be applied as pharmaceuticals or as ligands for catalysts, is accessible by copper-catalyzed oxidation with atmospheric oxygen. The scheme shows, for example, the synthesis of imidazo[1,5-a]pyridines 2 (R1=2-hydroxy-3-methoxyphenyl, tBu, 2-hydroxyphenyl, 2-aminophenyl; R2=2-pyridyl, Me, phenyl).
Supporting Information
Supporting information for this article is available on the WWW under http://www.wiley-vch.de/contents/jc_2002/2002/z18417_s.pdf or from the author.
Please note: The publisher is not responsible for the content or functionality of any supporting information supplied by the authors. Any queries (other than missing content) should be directed to the corresponding author for the article.
References
- 1
- 1aE. I. Solomon, U. M. Sundaram, T. E. Machonkin, Chem. Rev. 1996, 96, 2563;
- 1bE. I. Solomon, P. Chen, M. Metz, S.-K. Lee, A. E. Palmer, Angew. Chem. 2001, 113, 4702;
10.1002/1521-3757(20011217)113:24<4702::AID-ANGE4702>3.0.CO;2-6 Google ScholarAngew. Chem. Int. Ed. 2001, 40, 4570;10.1002/1521-3773(20011217)40:24<4570::AID-ANIE4570>3.0.CO;2-4 CAS PubMed Web of Science® Google Scholar
- 1cP. Gamez, P. G. Aubel, W. L. Driessen, J. Reedijk, Chem. Soc. Rev. 2001, 30, 376.
- 2P. Chaudhuri, M. Hess, U. Flörke, K. Wieghardt, Angew. Chem. 1998, 110, 2340;
10.1002/(SICI)1521-3757(19980817)110:16<2340::AID-ANGE2340>3.0.CO;2-C Google ScholarAngew. Chem. Int. Ed. 1998, 37, 2217.10.1002/(SICI)1521-3773(19980904)37:16<2217::AID-ANIE2217>3.0.CO;2-D CAS PubMed Web of Science® Google Scholar
- 3
- 3aK. D. Karlin, J. C. Hayes, Y. Gultneh, R. W. Cruse, J. W. McKown, J. P. Hutchinson, J. Zubieta, J. Am. Chem. Soc. 1984, 106, 2121;
- 3bI. E. Markó, P. R. Giles, M. Tsukazaki, S. M. Brown, C. J. Urch, Science 1996, 274, 2044.
- 4A. G. J. Ligtenbarg, A. L. Spek, R. Hage, B. L. Feringa, J. Chem. Soc. Dalton Trans. 1999, 659.
- 5P. Gómez-Saiz, J. Garciá-Tojal, M. A. Maestro, F. J. Arnaiz, T. Rojo, Inorg. Chem. 2002, 41, 1345.
- 6
- 6aM. Döring, M. Ciesielski, H. Görls, J. Inorg. Biochem. 1999, 74, 117;
- 6bM. Ciesielski, O. Walter, M. Döring, J. Inorg. Biochem. 2001, 86, 475.
- 7E. Niemers, R. Hiltmann, Synthesis 1976, 593.
- 8No additional base is necessary if R1 in 1 g is a heterocyclic fragment.
- 9
- 9aJ. D. Bower, G. R. Ramage, J. Chem. Soc. 1955, 2834;
- 9bK. Winterfeld, H. Franzke, Angew. Chem. 1963, 22, 1101; Angew. Chem. Int. Ed. Engl. 1963, 2, 736;
- 9cE. Abushanab, Tetrahedron Lett. 1971, 18, 1441;
10.1016/S0040-4039(01)96732-2 Google Scholar
- 9dM. T. Edgar, G. R. Pettit, T. S. Krupa, J. Org. Chem. 1979, 44, 396;
- 9eR. Tachikawa, S. Tanaka, A. Terada, Heterocycles 1981, 15( 1), 369;
- 9fA. P. Katritzky, C. W. Rees in Comprehensive Heterocyclic Chemistry, Vol. 5 (Ed ), Elsevier Science, Oxford, 1984, S. 634;
- 9gA. P. Krapcho, J. R. Powell, Tetrahedron Lett. 1986, 27, 3713;
- 9hM. Renz, C. Hemmert, B. Donnadieu, B. Meunier, Chem. Commun. 1998, 1635.
- 10
- 10aV. M. Aryuzina, M. N. Shchukina, Khim. Geterotsikl. Soedin. 1968, 3, 506;
- 10bM. P. Kochergin, V. A. Lifanov, Khim. Geterotsikl. Soedin. 1994, 4, 490.
- 11H. Reimlinger, J. J. M. Vandewalle, W. R. F. Lingier, E. de Ruiter, Chem. Ber. 1975, 108, 3771.
- 12Crystal structure analyses: Data were collected on a Nonius Kappa CCD diffractometer (MoKα radiation, λ=0.71073 Å, φ and ω scans), at 183 K, up to θmax=27.44°. The structure was solved by direct methods, and refined by least-squares against F2 (SHELXTL V5.10); non-hydrogen atoms anisotropic, hydrogen atoms located and refined isotropically. 2 a: C19H15N3O2, Mr=317.34, monoclinic, space group P2(1)/n, a=10.183(1), b=9.179(2), c=16.393(3) Å, β=104.50(2)°, V=1483.4(4) Å3, Z=4, ρcalcd=1.421 Mg m−3; 3117 measured reflections, 3011 independent reflections (Rint=0.0382), 2370 parameters, R1=0.0416 (for reflections with I>2σ(I)), wR2=0.1085 (for all reflections), residual electron density 0.213/−0.214 e Å−3. 3: C16H15N5O, Mr=293.33, monoclinic, space group P2(1)/n, a=9.7170(9), b=7.1997(7), c=20.147(1) Å, β=98.953(5)°, V=1392.3(2) Å3, Z=4, ρcalcd=1.399 Mg m−3; 5001 measured reflections, 2805 independent reflections (Rint=0.0715), 2805 parameters, R1=0.0715 (for reflections with I>2σ(I)), wR2=0.1580 (for all reflections), residual electron density 0.195/−0.303 e Å−3. CCDC-172796 (2 a) and CCDC-172797 (3) contain the supplementary crystallographic data for this paper. These data can be obtained free of charge via www.ccdc.cam.ac.uk/conts/retrieving.html (or from the Cambridge Crystallographic Data Centre, 12, Union Road, Cambridge CB2 1EZ, UK; fax: (+44) 1223-336-033; or [email protected]).



41:16<>1.0.co;2-7.cover.gif)
