[ε-PMo12O36(OH)4{La(H2O)4}4]5+: The First ε-PMo12O40 Keggin Ion and Its Association with the Two-Electron-Reduced α-PMo12O40 Isomer
Pierre Mialane Dr.
Institut Lavoisier, IREM, UMR 8637 Université de Versailles Saint-Quentin 45 Avenue des Etats-Unis, 78035 Versailles (France) Fax: (+33) 1-39-25-43-81
Search for more papers by this authorAnne Dolbecq Dr.
Institut Lavoisier, IREM, UMR 8637 Université de Versailles Saint-Quentin 45 Avenue des Etats-Unis, 78035 Versailles (France) Fax: (+33) 1-39-25-43-81
Search for more papers by this authorLaurent Lisnard
Institut Lavoisier, IREM, UMR 8637 Université de Versailles Saint-Quentin 45 Avenue des Etats-Unis, 78035 Versailles (France) Fax: (+33) 1-39-25-43-81
Search for more papers by this authorAlain Mallard
Institut Lavoisier, IREM, UMR 8637 Université de Versailles Saint-Quentin 45 Avenue des Etats-Unis, 78035 Versailles (France) Fax: (+33) 1-39-25-43-81
Search for more papers by this authorJérôme Marrot Dr.
Institut Lavoisier, IREM, UMR 8637 Université de Versailles Saint-Quentin 45 Avenue des Etats-Unis, 78035 Versailles (France) Fax: (+33) 1-39-25-43-81
Search for more papers by this authorFrancis Sécheresse Prof.
Institut Lavoisier, IREM, UMR 8637 Université de Versailles Saint-Quentin 45 Avenue des Etats-Unis, 78035 Versailles (France) Fax: (+33) 1-39-25-43-81
Search for more papers by this authorPierre Mialane Dr.
Institut Lavoisier, IREM, UMR 8637 Université de Versailles Saint-Quentin 45 Avenue des Etats-Unis, 78035 Versailles (France) Fax: (+33) 1-39-25-43-81
Search for more papers by this authorAnne Dolbecq Dr.
Institut Lavoisier, IREM, UMR 8637 Université de Versailles Saint-Quentin 45 Avenue des Etats-Unis, 78035 Versailles (France) Fax: (+33) 1-39-25-43-81
Search for more papers by this authorLaurent Lisnard
Institut Lavoisier, IREM, UMR 8637 Université de Versailles Saint-Quentin 45 Avenue des Etats-Unis, 78035 Versailles (France) Fax: (+33) 1-39-25-43-81
Search for more papers by this authorAlain Mallard
Institut Lavoisier, IREM, UMR 8637 Université de Versailles Saint-Quentin 45 Avenue des Etats-Unis, 78035 Versailles (France) Fax: (+33) 1-39-25-43-81
Search for more papers by this authorJérôme Marrot Dr.
Institut Lavoisier, IREM, UMR 8637 Université de Versailles Saint-Quentin 45 Avenue des Etats-Unis, 78035 Versailles (France) Fax: (+33) 1-39-25-43-81
Search for more papers by this authorFrancis Sécheresse Prof.
Institut Lavoisier, IREM, UMR 8637 Université de Versailles Saint-Quentin 45 Avenue des Etats-Unis, 78035 Versailles (France) Fax: (+33) 1-39-25-43-81
Search for more papers by this authorGraphical Abstract
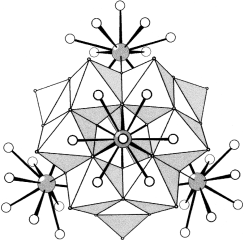
Sechs Mo-Dimere, die um ein zentrales PO4-Tetraeder gruppiert sind, bilden den Kern des neuartigen, von vier {La(H2O)4}3+-Einheiten umgebenen ε-Keggin-Polyoxokations [ε-PMo12O36(OH)4{La(H2O)4}4]5+ (siehe Struktur). 31P-NMR-spektroskopische Untersuchungen ergaben, dass ε-Keggin-Ionen in wässriger Lösung nicht stabil sind und ein Polyanion/Polykation-Salz bilden, das kristallographisch charakterisiert wurde.
References
- 1
- 1a Polyoxometalates: From Platonic solid to Anti-Retroviral Activity ( ), Kluwer Academic Publishers, Dordrecht, 1994;
- 1b M. T. Pope, A. Müller, Angew. Chem. 1991, 103, 56; Angew. Chem. Int. Ed. Engl. 1991, 30, 34;
- 1c Chem. Rev. 1998, 1–389 (Special issue on Polyoxometalates, Guest Ed. C. Hill).
- 2
- 2a See for example for the structure of [α-PMo12O40]3−: J. C. A. Boeyens, G. J. McDougal, J. Van, R. Smit, J. Solid State Chem. 1976, 18, 191;
- 2b for the structure of the one-electron-reduced anion: A. Proust, S. Taunier, V. Artero, F. Robert, R. Thouvenot, P. Gouzerh, Chem. Commun. 1996, 2195;
- 2c for the two-electron-reduced anion: R. Neier, C. Trojanowski, R. Mattes, J. Chem. Soc. Dalton Trans. 1995, 2521.
- 3 J. N. Barrows, G. B. Jameson, M. T. Pope, J. Am. Chem. Soc. 1985, 107, 1771.
- 4
- 4a G. Johansson, Ark. Kemi 1963, 20, 305;
- 4b G. Johansson, Ark. Kemi 1963, 20, 321;
- 4c W. O. Parker, R. Millini, I. Kiricsi, Inorg. Chem. 1997, 36, 571.
- 5 A. P. Lee, B. L. Philips, M. M. Olmstead, W. H. Casey, Inorg. Chem. 2001, 40, 4485.
- 6 H. Ichida, K. Nagai, Y. Sasaki, M. T. Pope, J. Am. Chem. Soc. 1989, 111, 586.
- 7 H. K. Chae, W. G. Klemperer, D. E. Paez Loyo, V. W. Day, T. A. Eberspacher, Inorg. Chem. 1992, 31, 3189.
- 8 M. I. Khan, A. Müller, S. Dillinger, H. Bögge, Q. Chen, J. Zubieta, Angew. Chem. 1993, 105, 1811; Angew. Chem. Int. Ed. Engl. 1993, 32, 1780.
- 9 M. I. Khan, Q. Chen, J. Salta, C. J. O'Connor, J. Zubieta, Inorg. Chem. 1996, 35, 1880.
- 10 A. Müller, C. Beugholt, P. Kögerler, H. Bögge, S. Bud'ko, M. Luban, Inorg. Chem. 2000, 39, 5176.
- 11
Crystal data and structure refinements for 1: a dark red cubic crystal (0.18×0.18×0.16 mm) was analyzed with a Siemens SMART three-circle diffractometer equipped with a CCD bidimensional detector using MoKα monochromatized radiation (λ=0.71073 Å). Cubic, space group P
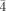 3m, a=12.2256(1) Å, V=1827.30(3) Å3, Z=1, ρcalcd=2.757 g cm−3, μ(MoKα)=7.105 mm−1, F(000)=1370, 13 173 reflections measured, of which 1030 were independent, 57 refined parameters, R=0.0572, wR2=0.1474. 2: a dark red crystal (0.34×0.22×0.22 mm); cubic, space group P
3m, a=12.2256(1) Å, V=1827.30(3) Å3, Z=1, ρcalcd=2.757 g cm−3, μ(MoKα)=7.105 mm−1, F(000)=1370, 13 173 reflections measured, of which 1030 were independent, 57 refined parameters, R=0.0572, wR2=0.1474. 2: a dark red crystal (0.34×0.22×0.22 mm); cubic, space group P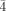 3m, a=12.4193(1) Å, V=1915.54(3) Å3, Z=1, ρcalcd=2.437 g cm−3, μ(MoKα)=4.344 mm−1, F(000)=1280, 8636 reflections measured, of which 990 were independent, 69 refined parameters, R=0.0701, wR2=0.1766. 3: a dark brown plate (0.14×0.12×0.04 mm); tetragonal, space group P
3m, a=12.4193(1) Å, V=1915.54(3) Å3, Z=1, ρcalcd=2.437 g cm−3, μ(MoKα)=4.344 mm−1, F(000)=1280, 8636 reflections measured, of which 990 were independent, 69 refined parameters, R=0.0701, wR2=0.1766. 3: a dark brown plate (0.14×0.12×0.04 mm); tetragonal, space group P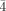 , a=20.2354(2), c=12.3785(2) Å, V=5068.6(1) Å3, Z=2, ρcalcd=3.182 g cm−3, μ(MoKα)=4.857 mm−1, F(000)=4460, 23 474 reflections measured, of which 7289 were independent, 399 refined parameters, R=0.0657, wR2=0.1248. All the molybdenum and phosphorus atoms of the Keggin ions were refined anisotropically while the potassium, oxygen, and chlorine atoms were refined by using isotropic temperature factors. For 1, 2, and 3, data reduction was performed with the SAINT software. The absorption correction was based on multiple and symmetry-equivalent reflections in the data set using the SADABS program based on the method of Blessing. The structures were solved by direct methods and refined by full-matrix least-squares using the SHELX-TL package. Further details on the crystal structure investigations may be obtained from the Fachinformationszentrum Karlsruhe, 76344 Eggenstein-Leopoldshafen, Germany (fax: (+49) 7247-808-666; e-mail: [email protected]), on quoting the depository numbers CSD-412375 (1), -412376 (2), and -412377 (3).
, a=20.2354(2), c=12.3785(2) Å, V=5068.6(1) Å3, Z=2, ρcalcd=3.182 g cm−3, μ(MoKα)=4.857 mm−1, F(000)=4460, 23 474 reflections measured, of which 7289 were independent, 399 refined parameters, R=0.0657, wR2=0.1248. All the molybdenum and phosphorus atoms of the Keggin ions were refined anisotropically while the potassium, oxygen, and chlorine atoms were refined by using isotropic temperature factors. For 1, 2, and 3, data reduction was performed with the SAINT software. The absorption correction was based on multiple and symmetry-equivalent reflections in the data set using the SADABS program based on the method of Blessing. The structures were solved by direct methods and refined by full-matrix least-squares using the SHELX-TL package. Further details on the crystal structure investigations may be obtained from the Fachinformationszentrum Karlsruhe, 76344 Eggenstein-Leopoldshafen, Germany (fax: (+49) 7247-808-666; e-mail: [email protected]), on quoting the depository numbers CSD-412375 (1), -412376 (2), and -412377 (3).
- 12 A. Müller, J. Meyer, E. Krickemeyer, E. Diemann, Angew. Chem. 1996, 108, 1296; Angew. Chem. Int. Ed. Engl. 1996, 35, 1206.
- 13
- 13a W. J. Evans, R. E. Golden, J. W. Ziller, Inorg. Chem. 1993, 32, 3041;
- 13b O. Poncelet, L. G. Hubert-Pfalzgraf, J.-C. Daran, R. Astier, J. Chem. Soc. Chem. Commun. 1989, 1846.
- 14 J.-M. Fruchart, P. Souchay, C. R. Hebd. Seances Acad. Sci. Ser. C 1968, 266, 1571.
- 15 N. E. Brese, M. O'Keeffe, Acta Crystallogr. Sect. B 1991, 47, 192.
- 16
- 16a A. Müller, E. Krickemeyer, M. Penk, V. Wittneben, J. Döring, Angew. Chem. 1990, 102, 85; Angew. Chem. Int. Ed. Engl. 1990, 29, 88;
- 16b N. Casañ-Pastor, P. Gomez-Romero, G. B. Jameson, L. C. W. Baker, J. Am. Chem. Soc. 1991, 113, 5658.
Citing Literature
This is the
German version
of Angewandte Chemie.
Note for articles published since 1962:
Do not cite this version alone.
Take me to the International Edition version with citable page numbers, DOI, and citation export.
We apologize for the inconvenience.



114:13<>1.0.co;2-j.cover.gif)
