Helical Sense Bias Induced by Point Chirality in Cage Compounds
Mateo Alajarín Dr.
Departamento de Química Orgánica Facultad de Química, Campus de Espinardo Universidad de Murcia, 30100 Murcia (Spain) Fax: (+349) 68-364149
Search for more papers by this authorCarmen López-Leonardo Dr.
Departamento de Química Orgánica Facultad de Química, Campus de Espinardo Universidad de Murcia, 30100 Murcia (Spain) Fax: (+349) 68-364149
Search for more papers by this authorAngel Vidal Dr.
Departamento de Química Orgánica Facultad de Química, Campus de Espinardo Universidad de Murcia, 30100 Murcia (Spain) Fax: (+349) 68-364149
Search for more papers by this authorJosé Berná
Departamento de Química Orgánica Facultad de Química, Campus de Espinardo Universidad de Murcia, 30100 Murcia (Spain) Fax: (+349) 68-364149
Search for more papers by this authorJonathan W. Steed Dr.
Department of Chemistry King's College London Strand, London WC2R 2LS (UK) Fax: (+44) 20-7848 2810
Search for more papers by this authorMateo Alajarín Dr.
Departamento de Química Orgánica Facultad de Química, Campus de Espinardo Universidad de Murcia, 30100 Murcia (Spain) Fax: (+349) 68-364149
Search for more papers by this authorCarmen López-Leonardo Dr.
Departamento de Química Orgánica Facultad de Química, Campus de Espinardo Universidad de Murcia, 30100 Murcia (Spain) Fax: (+349) 68-364149
Search for more papers by this authorAngel Vidal Dr.
Departamento de Química Orgánica Facultad de Química, Campus de Espinardo Universidad de Murcia, 30100 Murcia (Spain) Fax: (+349) 68-364149
Search for more papers by this authorJosé Berná
Departamento de Química Orgánica Facultad de Química, Campus de Espinardo Universidad de Murcia, 30100 Murcia (Spain) Fax: (+349) 68-364149
Search for more papers by this authorJonathan W. Steed Dr.
Department of Chemistry King's College London Strand, London WC2R 2LS (UK) Fax: (+44) 20-7848 2810
Search for more papers by this authorWe thank the Dirección General de Investigación-MCYT (Project BQU2001-0010), Fundación Séneca-CARM (Projects PB/2/FS/01 and PI-1/00749/FS/01), and Acedesa (a division of Takasago) for financial support. J.B. also thanks the MECD for a fellowship. J.W.S. thanks the EPSRC and King's College London for diffractometer funding.
Graphical Abstract
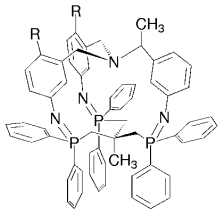
Chiralität für den Propeller: Bei der Herstellung von chiralen Käfigen mit Propeller-artigem Aufbau und Pseudo-C3-Symmetrie (siehe Bild; R=H, Cl) lässt sich durch Kontrolle der topologischen Asymmetrie ein ausgezeichneter Chiralitätstransfer erzielen. Zwei tripodale Einheiten werden durch drei P=N-Bindungen verknüpft, ein „Tripod-Tripod-Kupplungsprozess“, der von einer bemerkenswerten dreifachen Stickstoffabspaltung begleitet ist.
Supporting Information
Supporting information for this article is available on the WWW under http://www.angewandte.com or from the author.
Please note: The publisher is not responsible for the content or functionality of any supporting information supplied by the authors. Any queries (other than missing content) should be directed to the corresponding author for the article.
References
- 1 K. P. Meurer, F. Vögtle, Top. Curr. Chem. 1985, 127, 1; L. J. Prins, J. Huskens, F. De Jong, P. Timmerman, D. N. Reinhoudt, Nature 1999, 398, 498.
- 2 E. L. Eliel, S. H. Wilen, L. N. Mander, Stereochemistry of Organic Compounds, Wiley, New York, 1994, p. 1156.
- 3 K. Mislow, Acc. Chem. Res. 1976, 9, 26; K. Mislow, D. Gust, P. Finocchiaro, R. J. Boettcher, Fortschr. Chem. Forsch. 1974, 47, 1; K. P. Meurer, F. Vögtle, Top. Curr. Chem. 1985, 127, 1.
- 4
- 4a J.-M. Lehn, J.-P. Vigneron, Y. Bkouche–Waksman, J. Guilhem, C. Pascard, Helv. Chim. Acta 1992, 75, 1069;
- 4b M. Nakazaki, K. Yamamoto, T. Toya, J. Org. Chem. 1981, 46, 1611;
- 4c G. Hohner, F. Vögtle, Chem. Ber. 1977, 110, 3052;
- 4d R. P. L'Esperance, A. P. West, Jr., D. V. Engen, R. A. Pascal, Jr., J. Am. Chem. Soc. 1991, 113, 2672;
- 4e C. Bolm, K. B. Sharpless, Tetrahedron Lett. 1988, 29, 5101;
- 4f C. Bolm, W. D. Davis, R. L. Haltermann, K. B. Sharpless, Angew. Chem. 1988, 100, 882; Angew. Chem. Int. Ed. Engl. 1988, 27, 835.
- 5
- 5a
M. Alajarín, P. Molina, A. López–Lázaro, C. Foces–Foces, Angew. Chem. 1997, 109, 147;
10.1002/ange.19971090148 Google ScholarAngew. Chem. Int. Ed. Engl. 1997, 36, 67;
- 5b M. Alajarín, A. Vidal, C. López–Leonardo, J. Berná, Ramírez de Arellano, Tetrahedron Lett. 1998, 39, 7807;
- 5c
M. Alajarín, A. López–Lázaro, A. Vidal, J. Berná, Chem. Eur. J. 1998, 4, 2558.
10.1002/(SICI)1521-3765(19981204)4:12<2558::AID-CHEM2558>3.0.CO;2-3 CAS Web of Science® Google Scholar
- 6
The imination of tertiary phosphanes by azides, via intermediate phosphazides, to yield λ5-phosphazenes is known as the Staudinger PIII imination reaction: R
 P + R2N3→R
P + R2N3→R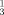 P=N−N=N−R2→R
P=N−N=N−R2→R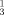 P=NR2 + N2. For references and reviews see: H. Staudinger, J. Meyer, Helv. Chim. Acta 1919, 2, 635;
Y. G. Gololobov, L. F. Kasukhin, Tetrahedron 1992, 48, 1353;
Y. G. Gololobov, Y. N. Zhmurova, L. F. Kasukhin, Tetrahedron 1981, 37, 437;
A. W. Johnson, Ylides and Imines of Phosphorus, Wiley, New York, 1993, p. 403. For recent high level calculations on the mechanism of the Staudinger PIII imination reaction see:
M. Alajarín, C. Conesa, H. S. Rzepa, J. Chem. Soc. Perkin Trans. 2 1999, 1811;
C. Widauer, H. Grützmacher, Y. Shevchenko, V. Gramlich, Eur. J. Inorg. Chem. 1999, 1659.
10.1002/(SICI)1099-0682(199910)1999:10<1659::AID-EJIC1659>3.0.CO;2-I CAS Web of Science® Google Scholar
P=NR2 + N2. For references and reviews see: H. Staudinger, J. Meyer, Helv. Chim. Acta 1919, 2, 635;
Y. G. Gololobov, L. F. Kasukhin, Tetrahedron 1992, 48, 1353;
Y. G. Gololobov, Y. N. Zhmurova, L. F. Kasukhin, Tetrahedron 1981, 37, 437;
A. W. Johnson, Ylides and Imines of Phosphorus, Wiley, New York, 1993, p. 403. For recent high level calculations on the mechanism of the Staudinger PIII imination reaction see:
M. Alajarín, C. Conesa, H. S. Rzepa, J. Chem. Soc. Perkin Trans. 2 1999, 1811;
C. Widauer, H. Grützmacher, Y. Shevchenko, V. Gramlich, Eur. J. Inorg. Chem. 1999, 1659.
10.1002/(SICI)1099-0682(199910)1999:10<1659::AID-EJIC1659>3.0.CO;2-I CAS Web of Science® Google Scholar - 7 New compounds have been fully characterized by spectroscopic methods and elemental composition (see Supporting Information).
- 8
Crystal structure analysis of 2 b⋅4 CHCl3: C66H58Br3Cl12N4P3, Mr=1665.20 g mol−1, triclinic space group P
 , a=12.8432(4), b=14.7484(6), c=19.5242(5) Å, α=85.839(2), β=89.058(2), γ=72.373(2)°, V=3515.2(2) Å3, Z=2, unique data 15 993 (2θ≤55°), parameters 795, R1 [F 2>2σ(F 2)] 0.044, wR2 (all data) 0.094. Crystal structure analysis of 4 b: C67.5H59Cl15.26N4P3, Mr=779.85 g mol−1, triclinic space group P
, a=12.8432(4), b=14.7484(6), c=19.5242(5) Å, α=85.839(2), β=89.058(2), γ=72.373(2)°, V=3515.2(2) Å3, Z=2, unique data 15 993 (2θ≤55°), parameters 795, R1 [F 2>2σ(F 2)] 0.044, wR2 (all data) 0.094. Crystal structure analysis of 4 b: C67.5H59Cl15.26N4P3, Mr=779.85 g mol−1, triclinic space group P , a=12.453(3), b=14.853(3), c=19.456(6) Å, α=85.522(14), β=89.845(15), γ=72.399(12)°, V=3419.0(15) Å3, Z=2, unique data 10 508 (2θ≤48°), parameters 796, R1 [F 2>2σ(F 2)] 0.190, wR2 (all data) 0.422. The poor overall precision resulted from the presence of a great deal of disordered chloroform, coupled with poor overall crystal quality as a result of decomposition. The structural details of the macrobicycle are clear and unambiguous, however. CCDC-143862 (2 b), and -172792 (4 b) contain the supplementary crystallographic data for this paper. These data can be obtained free of charge via www.ccdc.cam.ac.uk/conts/retrieving.html (or from the Cambridge Crystallographic Data Centre, 12, Union Road, Cambridge CB2 1EZ, UK; fax: (+44) 1223-336-033; or [email protected]).
, a=12.453(3), b=14.853(3), c=19.456(6) Å, α=85.522(14), β=89.845(15), γ=72.399(12)°, V=3419.0(15) Å3, Z=2, unique data 10 508 (2θ≤48°), parameters 796, R1 [F 2>2σ(F 2)] 0.190, wR2 (all data) 0.422. The poor overall precision resulted from the presence of a great deal of disordered chloroform, coupled with poor overall crystal quality as a result of decomposition. The structural details of the macrobicycle are clear and unambiguous, however. CCDC-143862 (2 b), and -172792 (4 b) contain the supplementary crystallographic data for this paper. These data can be obtained free of charge via www.ccdc.cam.ac.uk/conts/retrieving.html (or from the Cambridge Crystallographic Data Centre, 12, Union Road, Cambridge CB2 1EZ, UK; fax: (+44) 1223-336-033; or [email protected]).
- 9 The conventional assignment of the stereochemical descriptor P or M (helical twist sense) to the propeller units of the chiral macrobicycles was made by looking at the molecule along its threefold axis from the side of the tribenzylamine fragment.
- 10 The activation energy was calculated from the equation: ΔG≠=(4.57×10−3) Tc (10.32 + log(Tc2/πΔν)) following: D. H. Williams, I. Fleming, Spectroscopic Methods in Organic Chemistry, 4th ed., McGraw-Hill, New York, 1989, p. 103.
- 11 H. Gilboa, J. Altman, A. Loewenstein, J. Am. Chem. Soc. 1969, 91, 6062.
- 12 R. W. Alder, R. J. Arrowsmith, J. Chem. Res. (S) 1980, 163.
- 13 Compounds 2 did not hydrolize on heating solutions in a mixture of THF:H2O (5:1, v:v) at 60 °C for 48 h, monitored by 1H and 31P{1H} NMR.
- 14 Macrobicyclic cryptands with two intracyclic P=N units have been characterized, see: J. Mitjaville, A.-M. Caminade, R. Mathieu, J.-P. Majoral, J. Am. Chem. Soc. 1994, 116, 5007. For a review on bicyclic phosphorus compounds see: A.-M. Caminade, R. Kraemer, J.-P. Majoral, New J. Chem. 1997, 21, 627.
- 15 A.-M. Caminade, J.-P. Majoral, Chem. Rev. 1994, 94, 1183.
- 16 A comparable situation has been found in the tricyclic 2-methyl-1-azonia[4.4.4]propellane cation, see: J. M. McIntosh, J. Org. Chem. 1982, 47, 3777.
- 17 Semi-empirical calculations were carried out by using the MNDO method as implemented in the Gaussian 98 set of programs. The geometries of both stationary points were optimized, and harmonic frequency calculations were performed in order to characterize both structures as minima.
- 18 T. Mizutani, S. Yagi, T. Morinaga, T. Nomura, T. Takagishi, S. Kitagawa, H. Ogoshi, J. Am. Chem. Soc. 1999, 121, 754; J. W. Canary, C. S. Allen, J. M. Castagnetto, Y. Wang, J. Am. Chem. Soc. 1995, 117, 8484; Y. Yao, C. J. A. Daley, R. McDonald, S. H. Bergens, Organometallics 1997, 16, 1890.
- 19
L. J. Prins, J. Husken, F. De Jong, P. Timmerman, D. N. Reinhoudt, Nature 1999, 398, 498;
D. B. Amabilino, E. Ramos, J.-L. Serrano, T. Sierra, J. Veciana, J. Am. Chem. Soc. 1998, 120, 9126;
R. B. Prince, L. Brunsveld, E. W. Meijer, J. S. Moore, Angew. Chem. 2000, 112, 234;
10.1002/(SICI)1521-3757(20000103)112:1<234::AID-ANGE234>3.0.CO;2-6 Google ScholarAngew. Chem. Int. Ed. 2000, 39, 228.10.1002/(SICI)1521-3773(20000103)39:1<228::AID-ANIE228>3.0.CO;2-B CAS PubMed Web of Science® Google Scholar
Citing Literature
This is the
German version
of Angewandte Chemie.
Note for articles published since 1962:
Do not cite this version alone.
Take me to the International Edition version with citable page numbers, DOI, and citation export.
We apologize for the inconvenience.



114:7<>1.0.co;2-b.cover.gif)
