Dynamic Kinetic Resolution and Desymmetrization of Enantiotopic Groups by Cyclodehydration of Racemic or Prochiral δ-Oxoesters with (R)-Phenylglycinol: Enantioselective Synthesis of Piperidines
Mercedes Amat Prof. Dr.
Laboratory of Organic Chemistry, Faculty of Pharmacy University of Barcelona Av. Joan XXIII s/n, 08028-Barcelona (Spain) Fax: (+34) 93-402-45-39
Search for more papers by this authorMargalida Cantó Dr.
Laboratory of Organic Chemistry, Faculty of Pharmacy University of Barcelona Av. Joan XXIII s/n, 08028-Barcelona (Spain) Fax: (+34) 93-402-45-39
Search for more papers by this authorNúria Llor Dr.
Laboratory of Organic Chemistry, Faculty of Pharmacy University of Barcelona Av. Joan XXIII s/n, 08028-Barcelona (Spain) Fax: (+34) 93-402-45-39
Search for more papers by this authorViviana Ponzo Dr.
Laboratory of Organic Chemistry, Faculty of Pharmacy University of Barcelona Av. Joan XXIII s/n, 08028-Barcelona (Spain) Fax: (+34) 93-402-45-39
Search for more papers by this authorMaria Pérez
Laboratory of Organic Chemistry, Faculty of Pharmacy University of Barcelona Av. Joan XXIII s/n, 08028-Barcelona (Spain) Fax: (+34) 93-402-45-39
Search for more papers by this authorJoan Bosch Prof. Dr.
Laboratory of Organic Chemistry, Faculty of Pharmacy University of Barcelona Av. Joan XXIII s/n, 08028-Barcelona (Spain) Fax: (+34) 93-402-45-39
Search for more papers by this authorMercedes Amat Prof. Dr.
Laboratory of Organic Chemistry, Faculty of Pharmacy University of Barcelona Av. Joan XXIII s/n, 08028-Barcelona (Spain) Fax: (+34) 93-402-45-39
Search for more papers by this authorMargalida Cantó Dr.
Laboratory of Organic Chemistry, Faculty of Pharmacy University of Barcelona Av. Joan XXIII s/n, 08028-Barcelona (Spain) Fax: (+34) 93-402-45-39
Search for more papers by this authorNúria Llor Dr.
Laboratory of Organic Chemistry, Faculty of Pharmacy University of Barcelona Av. Joan XXIII s/n, 08028-Barcelona (Spain) Fax: (+34) 93-402-45-39
Search for more papers by this authorViviana Ponzo Dr.
Laboratory of Organic Chemistry, Faculty of Pharmacy University of Barcelona Av. Joan XXIII s/n, 08028-Barcelona (Spain) Fax: (+34) 93-402-45-39
Search for more papers by this authorMaria Pérez
Laboratory of Organic Chemistry, Faculty of Pharmacy University of Barcelona Av. Joan XXIII s/n, 08028-Barcelona (Spain) Fax: (+34) 93-402-45-39
Search for more papers by this authorJoan Bosch Prof. Dr.
Laboratory of Organic Chemistry, Faculty of Pharmacy University of Barcelona Av. Joan XXIII s/n, 08028-Barcelona (Spain) Fax: (+34) 93-402-45-39
Search for more papers by this authorThis work was supported by the DGICYT, Spain (BQU2000-0651), and the CUR, Generalitat de Catalunya (2001SGR-0084). We also thank the Ministry of Education, Culture, and Sport for fellowships to M.C. and M.P., as well as the CICYT, Spain, for a postdoctoral fellowship to V.P.
Graphical Abstract
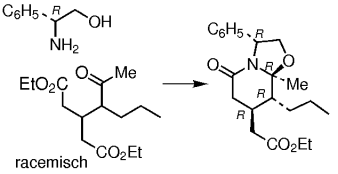
Enantiotope Estergruppen werden desymmetrisiert bei der Cyclodehydrierung prochiraler δ-Oxodiester mit (R)-Phenylglycinol. Diese Reaktion lässt sich auch auf racemische δ-Oxodiester erweitern, die in einem Tandemprozess aus dynamischer kinetischer Racematspaltung und diastereoselektiver Differenzierung reagieren (siehe Schema). Die erhaltenen bicyclischen Lactame sind präparativ nützlich für die Herstellung pharmakologisch bedeutsamer enantiomerenreiner Piperidinderivate.
Supporting Information
Supporting information for this article is available on the WWW under http://www.angewandte.com or from the author.
Please note: The publisher is not responsible for the content or functionality of any supporting information supplied by the authors. Any queries (other than missing content) should be directed to the corresponding author for the article.
References
- 1
- 1a M. Ohno, M. Otsuka, Org. React. 1989, 37, 1–55;
- 1b E. Schoffers, A. Golebiowski, C. R. Johnson, Tetrahedron 1996, 52, 3769–3826.
- 2 For reviews, see:
- 2a R. S. Ward, Chem. Soc. Rev. 1990, 19, 1–19;
- 2b S. R. Magnuson, Tetrahedron 1995, 51, 2167–2213;
- 2c M. C. Willis, J. Chem. Soc. Perkin Trans. 1 1999, 1765–1784.
- 3
- 3a R. Noyori, M. Tokunaga, M. Kitamura, Bull. Chem. Soc. Jpn. 1995, 68, 36–56;
- 3b R. S. Ward, Tetrahedron: Asymmetry 1995, 6, 1475–1490;
- 3c S. Caddick, K. Jenkins, Chem. Soc. Rev. 1996, 25, 447–456.
- 4
- 4a M. Amat, J. Bosch, J. Hidalgo, M. Cantó, M. Pérez, N. Llor, E. Molins, C. Miravitlles, M. Orozco, J. Luque, J. Org. Chem. 2000, 65, 3074–3084;
- 4b M. Amat, M. Pérez, N. Llor, J. Bosch, E. Lago, E. Molins, Org. Lett. 2001, 3, 611–614, and previous papers in this series.
- 5 The reactions were carried out by heating a solution of the δ-oxoacid derivative and (R)-phenylglycinol in toluene at reflux: D. Romo, A. I. Meyers, Tetrahedron 1991, 47, 9503–9569.
- 6 For the preparation of the starting δ-oxoacid derivatives, see Supporting Information.
- 7 The isomers were separated by means of column chromatography and gave satisfactory analytical and/or spectroscopic data.
- 8 For related examples which lead to tricyclic five-membered lactams, see:
- 8a J. A. Ragan, M. C. Claffey, Heterocycles 1995, 41, 57–70;
- 8b M. D. Ennis, R. L. Hoffman, N. B. Ghazal, D. W. Old, P. A. Mooney, J. Org. Chem. 1996, 61, 5813–5817.
- 9 For mechanistic considerations in related cyclodehydrations from unsubstituted ketoacids, see: A. I. Meyers, S. V. Downing, M. J. Weiser, J. Org. Chem. 2001, 66, 1413–1419.
- 10 Similar differences in stereoselectivity between unsubstituted aldehyde[4a] and ketoacid derivatives have been observed in related cyclodehydrations:
- 10a A. A. Bahajaj, P. D. Bailey, M. H. Moore, K. M. Morgan, J. M. Vernon, J. Chem. Soc. Chem. Commun. 1994, 2511–2512;
- 10b M. J. Munchhof, A. I. Meyers, J. Org. Chem. 1995, 60, 7084–7085;
- 10c N. Yamazaki, T. Ito, C. Kibayashi, Tetrahedron Lett. 1999, 40, 739–742.
- 11 H. Zarrinmayeh, A. M. Nunes, P. L. Ornstein, D. M. Zimmerman, M. B. Arnold, D. A. Schober, S. L. Gackenheimer, R. F. Bruns, P. A. Hipskind, T. C. Britton, B. E. Cantrell, D. R. Gehlert, J. Med. Chem. 1998, 41, 2709–2719.
- 12 For reviews, see:
- 12a T. Fujii, M. Ohba, Heterocycles 1988, 27, 1009–1033;
- 12b T. Fujii, M. Ohba, Heterocycles 1998, 47, 525–539.
- 13 Drugs Future 1987, 12, 88–89.
- 14 For reviews, see:
- 14a A. I. Meyers, G. P. Brengel, Chem. Commun. 1997, 1–8;
- 14b A. I. Meyers, C. J. Andres, J. E. Resek, C. C. Woodall, M. A. McLaughlin, P. H. Lee, D. A. Price, Tetrahedron 1999, 55, 8931–8952;
- 14c M. D. Groaning, A. I. Meyers, Tetrahedron 2000, 56, 9843–9873.
- 15 For reviews, see:
- 15a P. D. Bailey, P. A. Millwood, P. D. Smith, Chem. Commun. 1998, 633–640;
- 15b S. Laschat, T. Dickner, Synthesis 2000, 1781–1813.
Citing Literature
This is the
German version
of Angewandte Chemie.
Note for articles published since 1962:
Do not cite this version alone.
Take me to the International Edition version with citable page numbers, DOI, and citation export.
We apologize for the inconvenience.



114:2<>1.0.co;2-a.cover.gif)
