A Perfluorinated Nanosphere: Synthesis and Structure of Perfluoro-deca-B-methyl-para-carborane
Axel Herzog Dr.
Department of Chemistry and Biochemistry University of California, Los Angeles CA 90095-1569 (USA) Fax: (+1) 310-825-5490
Search for more papers by this authorRyan P. Callahan
Department of Chemistry and Biochemistry University of Texas at Austin Austin, TX, 78712 (USA) Fax: (+1) 512-471-8648
Search for more papers by this authorCharles L. B. Macdonald Dr.
Department of Chemistry and Biochemistry University of Texas at Austin Austin, TX, 78712 (USA) Fax: (+1) 512-471-8648
Search for more papers by this authorVincent M. Lynch Dr.
Department of Chemistry and Biochemistry University of Texas at Austin Austin, TX, 78712 (USA) Fax: (+1) 512-471-8648
Search for more papers by this authorM. Frederick Hawthorne Prof. Dr.
Department of Chemistry and Biochemistry University of California, Los Angeles CA 90095-1569 (USA) Fax: (+1) 310-825-5490
Search for more papers by this authorRichard J. Lagow Prof. Dr.
Department of Chemistry and Biochemistry University of Texas at Austin Austin, TX, 78712 (USA) Fax: (+1) 512-471-8648
Search for more papers by this authorAxel Herzog Dr.
Department of Chemistry and Biochemistry University of California, Los Angeles CA 90095-1569 (USA) Fax: (+1) 310-825-5490
Search for more papers by this authorRyan P. Callahan
Department of Chemistry and Biochemistry University of Texas at Austin Austin, TX, 78712 (USA) Fax: (+1) 512-471-8648
Search for more papers by this authorCharles L. B. Macdonald Dr.
Department of Chemistry and Biochemistry University of Texas at Austin Austin, TX, 78712 (USA) Fax: (+1) 512-471-8648
Search for more papers by this authorVincent M. Lynch Dr.
Department of Chemistry and Biochemistry University of Texas at Austin Austin, TX, 78712 (USA) Fax: (+1) 512-471-8648
Search for more papers by this authorM. Frederick Hawthorne Prof. Dr.
Department of Chemistry and Biochemistry University of California, Los Angeles CA 90095-1569 (USA) Fax: (+1) 310-825-5490
Search for more papers by this authorRichard J. Lagow Prof. Dr.
Department of Chemistry and Biochemistry University of Texas at Austin Austin, TX, 78712 (USA) Fax: (+1) 512-471-8648
Search for more papers by this authorThis research was funded by the National Science Foundation (USA) (NSF CHE 9314037) and (NSF CHE 9972888). We gratefully thank graduate student Theodore W. Bitner and Prof. Jeffrey I. Zink, UCLA, for the acquisition of the Raman spectra.
Graphical Abstract
References
- 1 Dictionary of Inorganic Compounds, 1st ed., (Ed.: J. E. Macintyre), Chapman & Hall, London, New York, 1992.
- 2 W. H. Knoth, H. C. Miller, D. C. England, G. W. Parshall, E. L. Muetterties, J. Am. Chem. Soc. 1962, 84, 1056–1057.
- 3 S. Kongpricha, H. Schroeder, Inorg. Chem. 1969, 8, 2449–2452.
- 4 R. J. Lagow, J. L. Margrave, J. Inorg. Nucl. Chem. 1973, 35, 2084–2085.
- 5 W. H. Knoth, H. C. Miller, J. C. Sauer, J. H. Balthis, V. T. Chia, E. L. Muetterties, Inorg. Chem. 1964, 3, 159–167.
- 6 S. V. Ivanov, J. J. Rockwell, O. G. Polyakov, C. M. Gaudinski, O. P. Anderson, K. A. Solntsev, S. H. Strauss, J. Am. Chem. Soc. 1998, 120, 4224–4225.
- 7 H. W. Kroto, J. R. Heath, S. C. O'Brien, R. F. Curl, R. E. Smalley, Nature 1985, 318, 162–163.
- 8 O. V. Boltalina, J. Fluorine Chem. 2000, 101, 273–278, and references therein.
- 9
- 9a A. A. Tuinman, A. A. Gakh, J. L. Adcock, R. N. Compton, J. Am. Soc. Chem. 1993, 115, 5885–5886;
- 9b A. A. Gakh, A. A. Tuinman, J. L. Adcock, R. A. Sachleben, R. N. Compton, J. Am. Soc. Chem. 1994, 116, 819–820.
- 10 A. A. T. Tuinman, P. Mukherjee, J. L. Adcock, R. L. Hettich, R. N. Compton, J. Phys. Chem. 1992, 96, 7584–7589.
- 11
- 11a R. Taylor, A. G. Avent, T. J. Dennis, J. P. Hare, H. W. Kroto, D. R. M. Walton, Nature 1992, 355, 27–29;
- 11b R. Taylor, J. A. Holloway, E. G. Hope, A. G. Avent, G. J. Langley, T. J. Dennis, J. P. Hare, H. W. Kroto, D. R. M. Walton, J. Chem. Soc. Chem. Commun. 1992, 665–668;
- 11c K. Kniaz, J. E. Fischer, H. Selig, G. B. M. Vaughan, W. J. Romanow, D. M. Cox, S. K. Chowdhury, J. P. McCauley, R. M. Strongin, A. B. Smith III, J. Am. Chem. Soc. 1991, 113, 6060–6064.
- 12
- 12a P. J. Fagan, P. J. Krusic, C. N. McEwen, J. Lazar, D. Holmes-Parker, N. Herron, E. Wasserman, Science 1993, 262, 404–407;
- 12b H. P. Fritz, R. Hiemeyer, Carbon 1995, 33, 1601–1609.
- 13 W. Jiang, C. B. Knobler, M. F. Hawthorne, Angew. Chem. 1996, 108, 2653–2655; Angew. Chem. Int. Ed. Engl. 1996, 35, 2536–2537.
- 14
A. Herzog, C. B. Knobler, M. F. Hawthorne, Angew. Chem. 1998, 37, 1552–1556;
10.1002/(SICI)1521-3773(19980619)37:11<1552::AID-ANIE1552>3.0.CO;2-J CAS PubMed Web of Science® Google ScholarAngew. Chem. Int. Ed. 1998, 110, 1661–1665.10.1002/(SICI)1521-3757(19980605)110:11<1661::AID-ANGE1661>3.0.CO;2-4 Web of Science® Google Scholar
- 15 B. King, J. Michl, J. Am. Chem. Soc. 2000, 122, 10 255–10 256.
- 16
- 16a W. Jiang, C. B. Knobler, M. D. Mortimer, M. F. Hawthorne, Angew. Chem. 1995, 107, 1470–1473; Angew. Chem. Int. Ed. Engl. 1995, 34, 1332–1334;
- 16b
A. Herzog, A. Maderna, G. N. Harakas, C. B. Knobler, M. F. Hawthorne, Chem. Eur. J. 1999, 5, 1212–1217.
10.1002/(SICI)1521-3765(19990401)5:4<1212::AID-CHEM1212>3.0.CO;2-W CAS Web of Science® Google Scholar
- 17 Scratching sublimed crystals with a metal spatula caused the material to detonate.
- 18
- 18a X-ray crystal data for 8: crystal dimensions: 0.2×0.2×0.16 mm, monoclinic, a=10.4450(5) Å, b=10.4002(5) Å, c=12.2536(6) Å, β=99.050(3)°, V=1314.54(11) Å3, space group P21/n, Z=2, formula: C12H2B10F30, Mr=824.21, ρcalcd=2.082 g cm−3, μ=0.266 mm−1; 5745 reflections were measured in the range 6.18°<2θ<54.98° on an Enraf Nonius KAPPA-CCD diffractometer (MoKα, graphite monochromator, T=−150 °C), 3012 unique after merging (Rint=0.0368). A correction was applied for Lorentzian polarization. The structure was solved by direct methods (SIR92) and refined by full-matrix least squares on F 2 using the Siemens SHELXL-97 (PC) software package. All non-hydrogen atoms were allowed anisotropic thermal motion. Number of parameters: 243. There appeared to be partial fluorine occupancy at the apical carbon atom of the molecule. Refinement with a hydrogen atom at the apical position resulted in a negative Uiso for the hydrogen atom. The C−H bond length was also longer than expected (1.15(3) Å). A possible explanation is the presence of a small percentage of fluorine which would increase the electron density at the apical position and have the effect of an elongated C−H bond. A model was generated where a partial occupancy fluorine atom and a partial occupancy hydrogen atom were refined. The sum of the site occupancy factors for the two atoms was fixed to be equal to 1. An isotropic displacement parameter was refined but constrained to be equal for the two atoms. The C−H and C−F bond lengths were constrained to be close to 0.96 Å and 1.30 Å, respectively. The B-C-H and B-C-F bond angles were kept nearly equal by constraining the five B-C-H contacts to be equivalent, at the same time the five B-C-F contacts were also constrained to be equal. In this way, the fluorine occupation was estimated to be 8(1) %. Final R factors: R1=0.1111, wR2=0.1798; max./min. residual electron density: 0.712/−0.284 e Å−3.
- 18b Crystallographic data (excluding structure factors) for the structures reported in this paper have been deposited with the Cambridge Crystallographic Data Centre as supplementary publication no. CCDC-153963 (8) and CCDC-153964 (5). Copies of the data can be obtained free of charge on application to CCDC, 12 Union Road, Cambridge CB2 1EZ, UK (fax: (+44) 1223-336-033; e-mail: [email protected]).
- 19 A. C. Rutenberg, A. A. Palko, J. Phys. Chem. 1965, 69, 527–531.
- 20 D. D. Callander, P. L. Coe, M. F. S. Matough, E. F. Mooney, A. J. Uff, P. H. Winson, J. Chem. Soc. Chem. Commun. 1966, 820–821.
- 21
X-ray crystal data for 5: crystal dimensions: 0.3×0.3×0.2 mm, triclinic, a=8.571(5), b=9.771(5), c=9.981(5) Å, α=117.601(5)°, β=113.317(5)°, γ=92.701(5)°, V=652.4(6) Å3, space group P
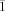 , Z=1, formula: C12B10F32, Mr=860.20, ρcalcd=2.190 g cm−3, μ=0.284 mm−1; 4446 reflections were measured in the range 6.18°<2θ<58.81° on an Enraf Nonius KAPPA-CCD diffractometer (MoKα, graphite monochromator, T=−20 °C), 2987 unique after merging (Rint=0.0343). A correction was applied for Lorentzian polarization. The structure was solved by direct methods (SIR92) and refined by full-matrix least squares on F 2 using SHELXL-93 in the WinGX software package. All non-hydrogen atoms were allowed anisotropic thermal motion. Number of parameters: 244. Final R factors: R1=0.1336, wR2=0.4164; max./min. residual electron density: 0.624/−0.682 e Å−3.[18b]
, Z=1, formula: C12B10F32, Mr=860.20, ρcalcd=2.190 g cm−3, μ=0.284 mm−1; 4446 reflections were measured in the range 6.18°<2θ<58.81° on an Enraf Nonius KAPPA-CCD diffractometer (MoKα, graphite monochromator, T=−20 °C), 2987 unique after merging (Rint=0.0343). A correction was applied for Lorentzian polarization. The structure was solved by direct methods (SIR92) and refined by full-matrix least squares on F 2 using SHELXL-93 in the WinGX software package. All non-hydrogen atoms were allowed anisotropic thermal motion. Number of parameters: 244. Final R factors: R1=0.1336, wR2=0.4164; max./min. residual electron density: 0.624/−0.682 e Å−3.[18b]
- 22 A. L. Henne, M. Nager, J. Am. Soc. Chem. 1952, 74, 650–652.
- 23 A. Herzog, T. J. Wedge, C. B. Knobler, M. F. Hawthorne, unpublished results.
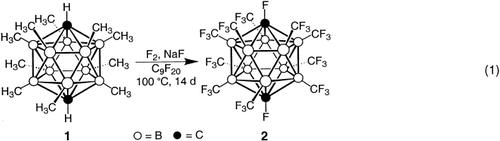
- 24 Preparation of 8, spectroscopic data for 1,12-(H)2-1,12-C2B10(CF3)10 (6) and 1-H-12-F-1,12-C2B10(CF3)10 (7). Caution!: Scraping the crystals that contained a mixture of compounds 5, 6, and 7 resulted in the detonation of those crystals[17] and extreme caution should be used in their handling. The reactor[25, 26] was filled with Freon 113 (300 mL) and NaF (16 g, 0.38 mol), cooled to −25 °C and purged with N2 (100 cm3 min−1) for 1 h. Compound 1 (250 mg, 0.88 mmol), dissolved in Freon 113 (150 mL), was pumped into the reactor (25 mL h−1), while a mixture of F2 and N2 was bubbled through the reactor (100 and 400 cm3 min−1, respectively). After the addition of the solution containing 1 was completed, the F2/N2 flow rate was reduced (25/25 cm3 min−1) and the temperature was gradually raised to 35 °C (72 h). The reaction was held at 35° C for an additional 10 h. The F2 was turned off and the reactor was allowed to purge (4 h). The contents of the reactor were filtered and all volatiles were removed under reduced pressure. Sublimation (110 °C/3 mmHg) furnished crystals containing compound 6, with small amounts of 5 and 7. Compound 6: 11B NMR (160.5 MHz, Freon 113, BF3⋅Et2O external): δ=−17.7 (s); 19F NMR (499.3 MHz, Freon 113, 5 % CDCl3): δ=−51.7 (d, 30 F, 2J(B,F)=55 Hz; CF3); 1H NMR (499.8 MHz, Freon 113, 5 % CDCl3): δ=5.1 (s); HR-MS (CI): m/z: calcd 826.0608, found 826.0586; compound 7: 11B NMR (160.5 MHz, Freon 113, BF3⋅Et2O external) δ=−15.8 (s), −17.7 (s); 19F NMR (499.3 MHz, Freon 113, 5 % CDCl3): δ=−49.1 (d, 30 F, 2J(B,F)=60 Hz, CF3), −51.7 (d, 30 F, 2J(B,F)=55 Hz, CF3), −157.5 (s, 1 F, CF); 1H NMR (499.8 MHz, Freon 113, 5 % CDCl3): δ=5.1 (s); HR-MS (CI): m/z: calcd 844.0514, found 844.0538.
- 25 R. J. Lagow, T. R. Bierschenk, T. J. Juhlke, J. Am. Chem. Soc. 1981, 103, 7340–7341.
- 26 T. R. Bierschenk, T. J. Juhlke, H. Kawa, R. J. Lagow, U.S. Patent 5 093 432, 1992.
- 27 The starting material 1 (neat) gives the following Raman data: 1/λ=180, 200, 366 (w), 266, 404, 421, 441 (s), 622, 792 (w), 1041 (w), 1321 (w), 1430 (br s, w) cm−1.
Citing Literature
This is the
German version
of Angewandte Chemie.
Note for articles published since 1962:
Do not cite this version alone.
Take me to the International Edition version with citable page numbers, DOI, and citation export.
We apologize for the inconvenience.



113:11<>1.0.co;2-#.cover.gif)
