Allyl Phenyl Sulfide†
Abstract
[5296-64-0] C9H10S (MW 150.24)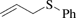
InChI = 1S/C9H10S/c1-2-8-10-9-6-4-3-5-7-9/h2-7H,1,8H2
InChIKey = QGNRLAFFKKBSIM-UHFFFAOYSA-N
(metalated derivatives are ambident nucleophiles; undergo reductive lithiation; precursors to sulfonium salts and sulfur ylides which undergo rearrangement)
Physical Data: bp 48–49 °C/0.43 mmHg; d 1.0220 g cm−3.
Solubility: insol water; sol most organic solvents.
Form Supplied in: colorless liquid of 98% purity as obtained commercially.
Preparative Method: from Allyl Bromide and sodium thiophenolate in ethanol.2
Purification: distillation in vacuo prior to use.
Handling, Storage, and Precautions: must be handled in a fume hood due to stench caused by contamination with Thiophenol. The pure sulfide has a more agreeable odor with a sweet overtone. No special precautions are necessary when handling in the atmosphere for weighing and transferring. In order to avoid autoxidation the sulfide should be stored in a well sealed vessel with exclusion of oxygen and moisture at 4 °C.



