Thiophenol
Onorato Campopiano
DuPont Agricultural Products, Wilmington, DE, USA
Search for more papers by this authorOnorato Campopiano
DuPont Agricultural Products, Wilmington, DE, USA
Search for more papers by this authorAbstract
[109-98-5] C6H6S (MW 110.19)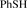
InChI = 1S/C6H6S/c7-6-4-2-1-3-5-6/h1-5,7H
InChIKey = RMVRSNDYEFQCLF-UHFFFAOYSA-N
(precursor of the thiophenoxy radical, which adds to alkenes and alkynes, initiating cyclizations; cis/trans alkene isomerization; synthesis of vinyl sulfides, alkyllithiums, pyridines; transacetalization; Michael addition)
Physical Data: mp −15 °C; bp 169 °C, 46.4 °C/4 mmHg; n20 1.5893; d20 1.0766 g cm−3.
Solubility: sol alcohol, ether, benzene, methylene chloride.
Form Supplied in: liquid; commercially available.
Handling, Storage, and Precautions: handle under an inert atmosphere as oxygen causes oxidation to the disulfide. This may accelerated in the presence of base. Stench reminiscent of garlic. Very toxic. Handle only in an efficient fume hood.
Bibliography
- 1 Miura, K.; Fugami, K.; Oshima, K.; Utimoto, K. Tetrahedron Lett. 1988, 29, 1543.
- 2 Miura, K.; Fugami, K.; Oshima, K.; Utimoto, K. Tetrahedron Lett. 1988, 29, 5135.
- 3 Broka, C. A.; Reichert, D. E. C. Tetrahedron Lett. 1987, 28, 1503.
- 4 Ichinose, Y.; Wakamatsu, K.; Nozaki, K.; Birbaum, J.-L.; Oshima, K.; Utimoto, K. Chem. Lett. 1987, 1647.
- 5 Schwarz, M.; Graminski, G. F.; Waters, R. M. J. Org. Chem. 1986, 51, 260.
- 6 Hopkins, P. B.; Fuchs, P. L. J. Org. Chem. 1978, 43, 1208.
- 7 See: Trost, B. M.; Lavoie, A. C. J. Am. Chem. Soc. 1983, 105, 5075, and references therein.
- 8 Screttas, C. G.; Screttas, M. M. J. Org. Chem. 1978, 43, 1064.
- 9 Cohen, T.; Lin, M.-T. J. Am. Chem. Soc. 1984, 106, 1130.
- 10 The first use of lithium di-t-butylbiphenylide for this purpose was on alkyl halides: Freeman, P. K.; Hutchinson, L. L. Tetrahedron Lett. 1976, 1849.
- 11 Bakuzis, P.; Bakuzis, M. L. F. J. Org. Chem. 1981, 46, 235.
- 12 Tanikaga, R.; Yamashita, H.; Kaji, A. Synthesis 1986, 416.
- 13 Bury, A.; Joag, S. D.; Stirling, C. J. M. Chem. Commun./J. Chem. Soc., Chem. Commun. 1986, 124.
- 14 Konno, K.; Hashimoto, K.; Shirahama, H.; Matsumoto, T. Tetrahedron Lett. 1986, 27, 3865.
- 15 Corey, E. J.; Ghosh, A. K. Tetrahedron Lett. 1988, 29, 3205.
- 16 Hackett, S.; Livinghouse, T. J. Org. Chem. 1986, 51, 879.
- 17 Kozikowski, A. P.; Ghosh, A. K. J. Org. Chem. 1985, 50, 3017.
- 18 Sinha, B.; Bose, J. L. Indian J. Chem., Sect. B 1991, 30, 340.
- 19 Nambiar, S.; Dacuble, J. F.; Doyle, R. J.; Taylor, K. G. Tetrahedron Lett. 1989, 30, 2179.
- 20 Julia, M.; Lefebvre, C. Tetrahedron Lett. 1984, 25, 189.
- 21 Gravel, D.; Farmer, L.; Ayotte, F. C. Tetrahedron Lett. 1990, 31, 63.
- 22 Kantorowski, E. J.; Eisenberg, S. W. E.; Fink, W. H.; Kurth, M. J., J. Org. Chem. 1999, 64, 570.
- 23 He, X.; Ortiz de Montellano, P. R., J. Org. Chem. 2004, 69, 5684.
- 24 Banwell, M. G.; Hockless, D. C. R.; McLeod, M. D., New J. Chem. 2003, 27, 50.
- 25 Gross, S.; Reissig, H.-U., Synlett 2002, 2027.
- 26 Majumdar, K. C.; Basu, P. K.; Mukhopadhyay, P. P., Tetrahedron 2004, 60, 6239 and references therein.
- 27 Busch-Petersen, J.; Hill, W. A.; Fan, P.; Khanolkar, A.; Xie, X.-Q.; Tius, M. A.; Makriyannis, A., J. Med. Chem. 1996, 39, 3790.
- 28 Al-awar, R. S.; Ray, J. E.; Schultz, R. M.; Andis, S. L.; Kennedy, J. H.; Moore, R. E.; Liang, J.; Golakoti, T.; Subbaraju, G. V.; Corbett, T. H., J. Med. Chem. 2003, 46, 2985.
- 29 Sugimura, T.; Nagano, S.; Tai, A., Tetrahedron Lett. 1997, 38, 3547.
- 30 Naito, T.; Honda, Y.; Miyata, O.; Ninomiya, I., J. Chem. Soc., Perkin Trans. 1 1995, 19.
- 31 Lamberto, M.; Corbett, D. F.; Kilburn, J. D., Tetrahedron Lett. 2003, 44, 1347.
- 32 Robertson, J.; Peplow, M. A.; Pillai, J., Tetrahedron Lett. 1996, 37, 5825.
- 33 Fukuyama, T.; Jow, C.-K.; Cheung, M., Tetrahedron Lett. 1995, 36, 6373.
- 34 Kurosawa, W.; Kan, T.; Fukuyama, T., Org. Synth. 2003, 79, 186.
- 35 Guisado, C.; Waterhouse, J. E.; Price, W. S.; Jorgensen, M. R.; Miller, A. D., Org. Biomol. Chem. 2005, 3, 1049.
- 36 Aurelio, L.; Brownlee, R. T. C.; Hughes, A. B., Chem. Rev. 2004, 104, 5823 and references therein.
- 37 Suzuki, M.; Kambe, M.; Tokuyama, H.; Fukuyama, T., J. Org. Chem. 2004, 69, 2831.
- 38 Burgett, A. W. G.; Li, Q.; Wei, Q.; Harran, P. G., Angew. Chem. Int. Ed. 2003, 42, 4961.
- 39 Tatsuta, K.; Imamura, K.; Itoh, S.; Kasai, S., Tetrahedron Lett. 2004, 45, 2847.
- 40 Monnee, M. C. F.; Brouwer, A. J.; Verbeek, L. M.; van Wageningen, A. M. A.; Liskamp, R. M. J., Bioorg. Med. Chem. Lett. 2001, 11, 1521.
- 41 Rew, Y.; Goodman, M., J. Org. Chem. 2002, 67, 8820.
- 42 Howland, R.; Gløgård, C.; Aasen, A. J.; Klaveness, J., Org. Biomol. Chem. 2003, 1, 644.
- 43 Skerlj, R. T.; Nan, S.; Zhou, Y.; Bridger, G. J., Tetrahedron Lett. 2002, 43, 7569.
- 44 Jabin, I.; Reinaud, O., J. Org. Chem. 2003, 68, 3416.
- 45 Basak, A.; Kar, M.; Mandal, S., Bioorg. Med. Chem. Lett. 2005, 15, 2061.
- 46 Stapper, C.; Blechert, S., J. Org. Chem. 2002, 67, 6456.
- 47 Brouwer, A. J.; Liskamp, R. M., J. Org. Chem. 2004, 69, 3662.
- 48 Fukuyama, T.; Cheung, M.; Jow, C.-K.; Hidai, Y.; Kan, T., Tetrahedron Lett. 1997, 38, 5831.
- 49 Nihei, K.-I.; Kato, M. J.; Yamane, T.; Palma, M. S.; Konno, K., Synlett 2001, 1167.
- 50 Cren, S.; Kinahan, T. C.; Skinner, C. L.; Tye, H., Tetrahedron Lett. 2002, 43, 2749.
- 51 Maligres, P. E.; See, M. M.; Askin, D.; Reider, P. J., Tetrahedron Lett. 1997, 38, 5253.
- 52 Otomaru, Y.; Tokunaga, N.; Shintani, R.; Hayashi, T., Org. Lett. 2005, 7, 307.
- 53 Okano, K.; Tokuyama, H.; Fukuyama, T., Org. Lett. 2003, 5, 4987.
- 54 Yamanaka, M.; Nishida, A.; Nakagawa, M., J. Org. Chem. 2003, 68, 3112.
- 55 Kay, C.; Murray, P. J.; Sandow, L.; Holmes, A. B., Tetrahedron Lett. 1997, 38, 6941.
- 56 Wills, A. J.; Cano, M.; Balasubramanian, S., J. Org. Chem. 2004, 69, 5439.
- 57 Ciolli, C. J.; Kalagher, S.; Belshaw, P. J., Org. Lett. 2004, 6, 1891.
- 58 Falkiewicz, B., Nucleosides, Nucleotides & Nucleic Acids 2002, 21, 883.
- 59 Virta, P.; Lönnberg, H., J. Org. Chem. 2003, 68, 8534.
- 60 Piró, J.; Rubiralta, M.; Giralt, E.; Diez, A., Tetrahedron Lett. 2001, 42, 871.
- 61 Wipf, P.; Henninger, T. C., J. Org. Chem. 1997, 62, 1586.
- 62 Wuts, P. G. M.; Gu, R. L.; Northuis, J. M.; Thomas, C. L., Tetrahedron Lett. 1998, 39, 9155.
- 63 Vedejs, E.; Kongkittingam, C., J. Org. Chem. 2001, 66, 7355.
- 64 Bartra, M.; Romea, P.; Urpi, F.; Vilarrasa, J., Tetrahedron 1990, 46, 587.
- 65 Baker, K. W. J.; March, A. R.; Parsons, S.; Paton, M. R.; Stewart, G. W., Tetrahedron 2002, 58, 8505.
- 66 Hammond, D. M.; Edmont, D.; Hornillo-Araujo, A. R.; Williams, D. M., Org. Biomol. Chem. 2003, 1, 4166.
- 67
Hughes, C. C.;
Trauner, D.,
Angew. Chem. Int. Ed.
2002,
41,
4556.
10.1002/1521-3773(20021202)41:23<4556::AID-ANIE4556>3.0.CO;2-E CAS PubMed Web of Science® Google Scholar
- 68
Ito, Y.;
Manabe, S.,
Chem. Eur. J.
2002,
8,
3076.
10.1002/1521-3765(20020715)8:14<3076::AID-CHEM3076>3.0.CO;2-N CAS PubMed Web of Science® Google Scholar
- 69 Bosse, F.; Marcaurelle, L. A.; Seeberger, P. H., J. Org. Chem. 2002, 67, 6659.
- 70 Coleman, R. S.; Felpin, F.-X.; Chen, W., J. Org. Chem. 2004, 69, 7309.
- 71
Jiang, J.;
Biggins, J. B.;
Thorson, J. S.,
Angew. Chem. Int. Ed.
2001,
40,
1502.
10.1002/1521-3773(20010417)40:8<1502::AID-ANIE1502>3.0.CO;2-K CAS PubMed Web of Science® Google Scholar
- 72 Lee, J. W.; Lu, J. Y.; Low, P. S.; Fuchs, P. L., Bioorg. Med. Chem. 2002, 10, 2397.
- 73 Blomberg, D.; Hedenström, M.; Kreye, P.; Sethson, I.; Brickmann, K.; Kihlberg, J., J. Org. Chem. 2004, 69, 3500.
- 74 Chino, M.; Wakao, M.; Ellmann, J. A., Tetrahedron 2002, 58, 6305.
- 75 Maltais, R.; Bérubé, M.; Marion, O.; Labrecque, R.; Poirier, D., Tetrahedron Lett. 2000, 41, 1691.
- 76 Kordalis, N. L.; Ioannou, P. V., Appl. Organomet. Chem. 2000, 14, 273.
- 77 Krow, G. R.; Lester, W. S.; Lin, G.; Fang, Y., J. Org. Chem. 2003, 68, 1626.
- 78 Fujihara, H.; Mima, H.; Furukawa, N., Tetrahedron 1996, 52, 10375.
- 79 Trost, B.; Krische, M. J., J. Am. Chem. Soc. 1999, 121, 6131.
- 80 Morel, G., Synlett 2003, 2167.
- 81 Furusho, Y.; Oku, T.; Hasegawa, T.; Tsuboi, A.; Kihara, N.; Takata, T., Chem. Eur. J. 2003, 9, 2895.
- 82 Praly, J.-P.; Ardakani, A. S.; Bruyére, I.; Marie-Luce, C.; Qin, B. B., Carbohydr. Res. 2002, 337, 1623.
- 83 Nayak, M. K.; Chakraborti, A. K., Tetrahedron Lett. 1997, 38, 8749.
- 84 Chakraborti, A. K.; Sharma, L.; Nayak, M. K., J. Org. Chem. 2002, 67, 2541.
- 85 Chakraborti, A. K.; Sharma, L.; Nayak, M. K., J. Org. Chem. 2002, 67, 6406.
- 86 Li, M.; Han, X.; Yu, B., J. Org. Chem. 2003, 68, 6842.
- 87 Smith, A. B., III; Adams, C. M.; Kozmin, S. A.; Paone, D. V., J. Am. Chem. Soc. 2001, 123, 5925.
- 88 Banerjee, M.; Mukhopadhyay, R.; Achari, B.; Banerjee, A. K., Org. Lett. 2003, 5, 3931.
- 89 Marques, M. A.; Doss, R. M.; Foister, S.; Dervan, P. B., J. Am. Chem. Soc. 2004, 126, 10339.
- 90 Martinot, T. A.; Benner, S. A., J. Org. Chem. 2004, 69, 3972.
- 91 Scribner, A. W.; Haroutounian, S. A.; Carlson, K. E.; Katzenellenbogen, J. A., J. Org. Chem. 1997, 62, 1043.
- 92 Kim, D.; Lee, J.; Shim, P. J.; Lim, J. I.; Jo, H.; Kim, S., J. Org. Chem. 2002, 67, 764.
- 93 Bröder, W.; Kunz, H., Bioorg. Med. Chem. 1997, 5, 1.
- 94 Fujishima, T.; Konno, K.; Nakagawa, K.; Kurobe, M.; Okano, T.; Takayama, H., Bioorg. Med. Chem. 2000, 8, 123.
- 95 Shiozaki, M., Carbohydr. Res. 2001, 335, 147.
- 96 Matsumura, R.; Suzuki, T.; Hagiwara, H.; Hoshi, T.; Ando, M., Tetrahedron Lett. 2001, 42, 1543.
- 97 Wéber, C.; Bielik, A.; Szendrei, G. I.; Greiner, I., Tetrahedron Lett. 2002, 43, 2971.
- 98 Beumer, R.; Bayón, P.; Bugada, P.; Ducki, S.; Mongelli, N.; Riccardi Sirtori, F.; Telser, J.; Gennari, C., Tetrahedron 2003, 59, 8803.
- 99 Shiozaki, M., Carbohydr. Res. 2002, 337, 2077.
- 100 Murakami, T.; Taguchi, K., Tetrahedron 1999, 55, 989.
- 101 Masaki, Y.; Tanaka, N.; Miura, T., Tetrahedron Lett. 1998, 39, 5799.
- 102 McLeod, C.; McKiernan, G. J.; Guthrie, E. J.; Farrugia, L. J.; Hamprecht, D. W.; Macritchie, J.; Hartley, R. C., J. Org. Chem. 2003, 68, 387.
- 103 Khan, A. T.; Mondal, E.; Sahu, P. R.; Islam, S., Tetrahedron Lett. 2003, 44, 919.
- 104 Fehr, C.; Galindo, J., Helv. Chim. Acta. 1995, 78, 539.
- 105 Chakraborti, A. K.; Nayak, M. K.; Sharma, L., J. Org. Chem. 1999, 64, 8027.
- 106 Li, M.; Han, X.; Yu, B., Tetrahedron Lett. 2002, 43, 9467.
- 107 Vassis, S.; Govaris, I.; Voyagi, K.; Mamos, P.; Papaioannou, D., Tetrahedron Lett. 2002, 43, 2597.
- 108 Daub, G. W.; vanTamelen, E. E., J. Am. Chem. Soc. 1977, 99, 3526.
- 109 Zhang, Y.; Heinsen, M. H.; Kostic, M.; Pagani, G. M.; Riera, T. V.; Perovic, I.; Hedstrom, L.; Snider, B. B.; Pochapsky, T. C., Bioorg. Med. Chem. 2004, 12, 3847.
- 110 Müller, B.; Martin, T. J.; Schaub, C.; Schmidt, R. R., Tetrahedron Lett. 1998, 39, 509.
- 111 Georgiadis, D.; Dive, V.; Yiotakis, A., J. Org. Chem. 2001, 66, 6604.
- 112 Borodkin, V. S.; Milne, F. C.; Ferguson, M. A. J.; Nikolaev, A. V., Tetrahedron Lett. 2002, 43, 7821.
- 113 Parang, K., Bioorg. Med. Chem. Lett. 2002, 12, 1863.
- 114 Shimizu, M.; Wada, T.; Oka, N.; Saigo, K., J. Org. Chem. 2004, 69, 5261.
- 115
Punniyamurthy, T.;
Irie, R.;
Katsuki, T.,
Chirality
2000,
12,
464.
10.1002/(SICI)1520-636X(2000)12:5/6<464::AID-CHIR28>3.0.CO;2-Z CAS PubMed Web of Science® Google Scholar
- 116
Rassu, G.;
Carta, P.;
Pinna, L.;
Battistini, L.;
Zanardi, F.;
Acquotti, D.;
Casiraghi, G.,
Eur. J. Org. Chem.
1999,
1395.
10.1002/(SICI)1099-0690(199906)1999:6<1395::AID-EJOC1395>3.0.CO;2-T CAS Web of Science® Google Scholar
- 117 Fattori, D.; Kinzel, O.; Ingallinella, P.; Bianchi, E.; Pessi, A., Bioorg. Med. Chem. Lett. 2002, 12, 1143.
- 118 Mitchell, H. J.; Warren, S., Tetrahedron Lett. 1996, 37, 2105.
- 119 Gruttadauria, M.; Lo Meo, P.; Noto, R., Tetrahedron 1999, 55, 14097.
- 120 Gravel, D.; Bordeleau, J., Tetrahedron Lett. 1998, 39, 8035.
- 121 Walford, C.; Jackson, R. F. W.; Rees, N. H.; Clegg, W.; Heath, S. L., Chem. Commun. 1997, 1855.
- 122 Raghavan, S.; Reddy, S. R., J. Org. Chem. 2003, 68, 5754.
- 123 Hirasawa, H.; Taniguchi, T.; Ogasawara, K., Tetrahedron Lett. 2001, 42, 7587.
- 124
Le Hetet, C.;
David, M.;
Carreaux, F.;
Carboni, B.;
Sauleau, A.,
Tetrahedron Lett.
1997,
38,
5153.
10.1016/S0040-4039(97)01098-8 Google Scholar
- 125 Cuadrado, P.; Gonzalez-Nogal, A. M., Tetrahedron Lett. 2000, 41, 1111.
- 126 Cuadrado, P.; Gonzalez-Nogal, A. M., Tetrahedron Lett. 2001, 42, 8993.
- 127 Fringuelli, F.; Pizzo, F.; Tortoioli, S.; Vaccaro, L., J. Org. Chem. 2003, 68, 8248.
- 128 Fringuelli, F.; Pizzo, F.; Tortoioli, S.; Vaccaro, L., Adv. Synth. Catal. 2002, 344, 379.
- 129 Aggarwal, V. K.; Hynd, G.; Picoul, W.; Vasse, J.-L., J. Am. Chem. Soc. 2002, 124, 9964.
- 130 Chandrasekhar, S.; Reddy, C. R.; Babu, B. N.; Chandrasekhar, G., Tetrahedron Lett. 2002, 43, 3801.
- 131 Fan, R.-H.; Hou, X.-L., J. Org. Chem. 2003, 68, 726.
- 132 Fringuelli, F.; Pizzo, F.; Tortoioli, S.; Vaccaro, L., Tetrahedron Lett. 2003, 44, 6785.
- 133 Bae, J. H.; Shin, S.-H.; Park, C. S.; Lee, W. K., Tetrahedron 1999, 55, 10041.
- 134 Dauban, P.; Dodd, R. H., Org. Lett. 2000, 2, 2327.
- 135 Watson, I. D. G.; Yudin, A. K., J. Org. Chem. 2003, 68, 5160.
- 136 Hou, X.-L.; Fan, R.-H.; Dai, L.-X., J. Org. Chem. 2002, 67, 5295.
- 137 Álvares, Y. S. P.; Alves, M. J.; Azoia, N. G.; Bickley, J. F.; Gilchrist, T. L., J. Chem. Soc., Perkin Trans. 1 2002, 1911.
- 138 Müller, P.; Riegert, D., Tetrahedron 2005, 61, 4373.
- 139 Takano, M.; Umino, A.; Nakada, M., Org. Lett. 2004, 6, 4897.
- 140 Taber, D. F.; Xu, M.; Hartnett, J. C., J. Am. Chem. Soc. 2002, 124, 13121.
- 141 Agami, C.; Amiot, F.; Couty, F.; Dechoux, L.; Kaminsky, C.; Venier, O., Tetrahedron: Asymmetry 1998, 9, 3955.
- 142 Ishibashi, H.; Uegaki, M.; Sakai, M., Synlett 1997, 915.
- 143 Albizati, K. F.; Babu, S.; Birchler, A.; Busse, J. K.; Fugett, M.; Grubbs, A.; Haddach, A.; Pagan, M.; Potts, B.; Remarchuk, T.; Rieger, D.; Rodriguez, R.; Shanley, J.; Szendroi, R.; Tibbetts, T.; Whitten, K.; Borer, B. C., Tetrahedron Lett. 2001, 42, 6481.
- 144 Zhang, Z.; Magnusson, G., Carbohydr. Res. 1996, 295, 41.
- 145
Nicolaou, K. C.;
Mitchell, H. J.;
Jain, N. F.;
Bando, T.;
Hughes, R.;
Winssinger, N.;
Natarajan, S.;
Koumbis, A. E.,
Chem. Eur. J.
1999,
5,
2648.
10.1002/(SICI)1521-3765(19990903)5:9<2648::AID-CHEM2648>3.0.CO;2-Q CAS Web of Science® Google Scholar
- 146 Barroca, N.; Schmidt, R. R., Org. Lett. 2004, 6, 1551.
- 147 Agarwal, A.; Rani, S.; Vankar, Y. D., J. Org. Chem. 2004, 69, 6137.
- 148 Yu, M.; Pagenkopf, B. L., Tetrahedron 2003, 59, 2765.
- 149 Fujiwara, K.; Kobayashi, M.; Yamamoto, F.; Aki, Y.-I.; Kawamura, M.; Awakura, D.; Amano, S.; Okano, A.; Murai, A.; Kawai, H.; Suzuki, T., Tetrahedron Lett. 2005, 46, 5067.
- 150 Cooksey, J.; Gunn, A.; Kocienski, P. J.; Kuhl, A.; Uppal, S.; Christopher, J. A.; Bell, R., Org. Biomol. Chem. 2004, 2, 1719.
- 151 Luzzio, F. A.; Mayorov, A. V.; Ng, S. S. W.; Kruger, E. A.; Figg, W. D., J. Med. Chem. 2003, 46, 3793.
- 152 Khim, S.-K.; Schultz, A. G., J. Org. Chem. 2004, 69, 7734.
- 153 Cousins, G.; Falshaw, A.; Hoberg, J. O., Org. Biomol. Chem. 2004, 2, 2272.
- 154 Wipf, P.; Spencer, S. R., J. Am. Chem. Soc. 2005, 127, 225.
- 155 Herzon, S. B.; Myers, A. G., J. Am. Chem. Soc. 2005, 127, 5342.
- 156 Bandini, M.; Cozzi, P. G.; Giacomini, M.; Melchiorre, P.; Selva, S.; Umani-Ronchi, A., J. Org. Chem. 2002, 67, 3700.
- 157 Bartoli, G.; Bartolacci, M.; Giuliani, A.; Marcantoni, E.; Massaccesi, M.; Torregiani, E., J. Org. Chem. 2005, 70, 169.
- 158 Raghavan, S.; Tony, K. A., J. Org. Chem. 2003, 68, 5002.
- 159 Chu, C.-M.; Gao, S.; Sastry, M. N. V.; Yao, C.-F., Tetrahedron Lett. 2005, 46, 4971.
- 160 Ito, A.; Konishi, K.; Aida, T., Tetrahedron Lett. 1996, 37, 2585.
- 161 Krishnaveni, N. S.; Surendra, K.; Rao, K. R., Chem. Commun. 2005, 669.
- 162 Skarzewski, J.; Zielinska-Blajet, M.; Turowska-Tyrk, I., Tetrahedron: Asymmetry 2001, 12, 1923.
- 163 Danelli, T.; Annunziata, R.; Benaglia, M.; Cinquini, M.; Cozzi, F.; Tocco, G., Tetrahedron: Asymmetry 2003, 14, 461.
- 164 Zielinska-Blajet, M.; Kowalczyk, R.; Skarzewski, J., Tetrahedron 2005, 61, 5235.
- 165 Glenn, A. G.; Jones, P. B., Tetrahedron Lett. 2004, 45, 6967.
- 166 Yadav, J. S.; Reddy, B. V. S.; Baishya, G., J. Org. Chem. 2003, 68, 7098.
- 167 Nava-Salgado, V. O.; Adam, W., Eur. J. Org. Chem. 2000, 2529.
- 168 Schneider, R.; Gerardin, P.; Loubinoux, B.; Rihs, G., Tetrahedron 1995, 51, 4997.
- 169 Bartels, B.; Clayden, J.; Gonzalez Martín, C.; Nelson, A.; Russell, M. G.; Warren, S., J. Chem. Soc., Perkin Trans. 1 1999, 1807.
- 170 Moriarty, R. M.; Enache, L. A.; Gilardi, R.; Gould, G. L.; Wink, D. J., Chem. Commun. 1998, 1155.
- 171 Wipf, P.; Graham, T. H., Org. Biomol. Chem. 2005, 3, 31.
- 172 Kraus, G. A.; Kim, I., J. Org. Chem. 2003, 68, 4517.
- 173 Zhu, S.; Cohen, T., Tetrahedron 1997, 53, 17607.
- 174 Boto, A.; Hernández, R.; Velázquez, S. M.; Suàrez, E., J. Org. Chem. 1998, 63, 4697.
- 175 Chen, F.; Mudryk, B.; Cohen, T., Tetrahedron 1999, 55, 3291.
- 176 Kamimura, A.; Omata, Y.; Mitsudera, H.; Kakehi, A., J. Chem. Soc., Perkin Trans. 1 2000, 4499.
- 177 Inomata, K.; Barragué, M.; Paquette, L. A., J. Org. Chem. 2005, 70, 533.
- 178 Clauss, R.; Hinz, W.; Hunter, R., Synlett 1997, 57.
- 179 Dawson, P. E.; Muir, T. W.; Clark-Lewis, I.; Kent, S. B. H., Science 1994, 266, 776.
- 180 Dawson, P. E.; Churchill, M. J.; Ghadiri, M. R.; Kent, S. B. H., J. Am. Chem. Soc. 1997, 119, 4325.
- 181 Kimmerlin, T.; Seebach, D.; Hilvert, D., Helv. Chim. Acta. 2002, 85, 1812.
- 182 Matsumura, S.; Takahashi, T.; Ueno, A.; Mihara, H., Chem. Eur. J. 2003, 9, 4829.
- 183 Galoníc, D. P.; Ide, N. D.; van derDonk, W. A.; Gin, D. Y., J. Am. Chem. Soc. 2005, 127, 7359.
- 184
deKoning, M. C.;
Filippov, D. V.;
Meeuwenoord, N.;
Overhand, M.;
van derMarel, G. A.;
van Boom, J. H.,
Synlett
2001,
1516.
10.1055/s-2001-17455 Google Scholar
- 185 Tanaka, Y.; Nakahara, Y.; Hojo, H.; Nakahara, Y., Tetrahedron 2003, 59, 4059.
- 186 Botti, P.; Villain, M.; Manganiello, S.; Gaertner, H., Org. Lett. 2004, 6, 4861.
- 187 Ollivier, N.; Behr, J.-B.; El-Mahdi, O.; Blanpain, A.; Melnyk, O., Org. Lett. 2005, 7, 2647.
- 188 Tulla-Puche, J.; Barany, G., J. Org. Chem. 2004, 69, 4101.
- 189 Tam, J. P.; Yu, Q.; Yang, J.-L., J. Am. Chem. Soc. 2001, 123, 2487.
- 190 Gieselman, M. D.; Xie, L.; van derDonk, W. A., Org. Lett. 2001, 3, 1331.
- 191 Yan, L. Z.; Dawson, P. E., J. Am. Chem. Soc. 2001, 123, 526.
- 192
Melander, C.;
Herman, D. M.;
Dervan, P. B.,
Chem. Eur. J.
2000,
6,
4487.
10.1002/1521-3765(20001215)6:24<4487::AID-CHEM4487>3.0.CO;2-C CAS PubMed Web of Science® Google Scholar
- 193 Valverde, M. G.; Dallinger, D.; Kappe, C. O., Synlett 2001, 741.
- 194 D'herde, J. N. P.; De Clercq, P. J., Tetrahedron Lett. 2003, 44, 6657.
- 195 Parrot, I.; Wermuth, C.-G.; Hibert, M., Tetrahedron Lett. 1999, 40, 7975.
- 196 Katoh, M.; Sodeoka, M., Bioorg. Med. Chem. Lett. 1999, 9, 881.
- 197 Ermann, M.; Simkovsky, N. M.; Roberts, S. M.; Parry, D. M.; Baxter, A. D.; Montana, J. G., Tetrahedron Lett. 2000, 41, 2483.
- 198 Pirrung, M. C.; Liu, H., Org. Lett. 2003, 5, 1983.
- 199 Shah, S. T. A.; Khan, K. M.; Heinrich, A. M.; Voelter, W., Tetrahedron Lett. 2002, 43, 8281.
- 200 Tundo, P.; Rossi, L.; Loris, A., J. Org. Chem. 2005, 70, 2219.
- 201 Smith, III, A. B.; Cho, Y. S.; Ishiyama, H., Org. Lett. 2001, 3, 3971.
- 202 Hagiwara, H.; Kobayashi, K.; Miya, S.; Hoshi, T.; Suzuki, T.; Ando, M.; Okamoto, T.; Kobayashi, M.; Yamamoto, I.; Ohtsubo, S.; Kato, M.; Uda, H., J. Org. Chem. 2002, 67, 5969.
- 203 Chauhan, S. M. S.; Kumar, A.; Srinivas, K. A., Chem. Commun. 2003, 2348.
- 204 Joshi, A. V.; Bhusare, S.; Baidossi, M.; Qafisheh, N.; Sasson, Y., Tetrahedron Lett. 2005, 46, 3583.
- 205 Bates, C. G.; Saejueng, P.; Doherty, M. Q.; Venkataraman, D., Org. Lett. 2004, 6, 5005.
- 206 Kwong, F. Y.; Buchwald, S. L., Org. Lett. 2002, 4, 3517.
- 207 Itoh, T.; Mase, T., Org. Lett. 2004, 6, 4587.
- 208 Han, L.-B.; Tanaka, M., Chem. Commun. 1999, 395.
- 209
El Ali, B.;
Tijani, J.;
El-Ghanam, A.;
Fettouhi, M.,
Tetrahedron Lett.
2001,
42,
1567.
10.1016/S0040-4039(00)02169-9 Google Scholar
- 210 Xiao, W.-J.; Alper, H., J. Org. Chem. 2005, 70, 1802.
- 211
vonSeebach, M.;
Grigg, R.;
deMeijere, A.,
Eur. J. Org. Chem.
2002,
3268.
10.1002/1099-0690(200210)2002:19<3268::AID-EJOC3268>3.0.CO;2-H Google Scholar
- 212 Hart, D. J.; Magomedov, N. A., J. Am. Chem. Soc. 2001, 123, 5892.
- 213 Phukan, P.; Mohan, J. M.; Sudalai, A., J. Chem. Soc., Perkin Trans. 1 1999, 3685.
- 214 Blake, A. J.; Friend, C. L.; Outram, R. J.; Simpkins, N. S.; Whitehead, A. J., Tetrahedron Lett. 2001, 42, 2877.
- 215 Leroux, F.; Jeschke, P.; Schlosser, M., Chem. Rev. 2005, 105, 827.
- 216 Zhdankin, V. V.; Mayadanovych, O.; Herschbach, J.; Bruno, J.; Matveeva, E. D.; Zefirov, N. S., J. Org. Chem. 2003, 68, 1018.
- 217 Katritzky, A. R.; Xu, Y.-J.; Jain, R., J. Org. Chem. 2002, 67, 8234.



