Biological concepts in human sodium channel epilepsies and their relevance in clinical practice
Corresponding Author
Andreas Brunklaus
Paediatric Neurosciences Research Group, Royal Hospital for Children, Glasgow, UK
School of Medicine, University of Glasgow, Glasgow, UK
Andreas Brunklaus, Juanjiangmeng Du and Dennis Lal contributed equally to this work
Correspondence
Andreas Brunklaus, Fraser of Allander Neurosciences Unit, Office Block, Ground Floor, Zone 2, Royal Hospital for Children, 1345 Govan Road, Glasgow G51 4TF, UK.
Email: [email protected]
Rikke S. Møller, Head of Department of Epilepsy Genetics and Personalized Medicine, Danish Epilepsy Centre/University of Southern Denmark, Medicine Kolonivej 1, 4293, Dianalund, Denmark.
Email: [email protected]
Dennis Lal, Genomic Medicine Institute, Cleveland Clinic Lerner College of Medicine of Case Western Reserve University, US, Epilepsy Institute, Cleveland Clinic, 9500 Euclid Ave, NE5-308, Cleveland, OH 44195, USA.
Emails: [email protected]; [email protected]
Search for more papers by this authorJuanjiangmeng Du
Cologne Center for Genomics, University of Cologne, University Hospital Cologne, Cologne, Germany
Andreas Brunklaus, Juanjiangmeng Du and Dennis Lal contributed equally to this work
Search for more papers by this authorFelix Steckler
Paediatric Neurosciences Research Group, Royal Hospital for Children, Glasgow, UK
School of Medicine, University of Glasgow, Glasgow, UK
Search for more papers by this authorIsmael I. Ghanty
Paediatric Neurosciences Research Group, Royal Hospital for Children, Glasgow, UK
School of Medicine, University of Glasgow, Glasgow, UK
Search for more papers by this authorKatrine M. Johannesen
Deparment of Epilepsy Genetics and Personalized Medicine, Danish Epilepsy Center Filadelfia, Dianalund, Denmark
Institute for Regional Health Services, University of Southern Denmark, Odense, Denmark
Search for more papers by this authorChristina Dühring Fenger
Deparment of Epilepsy Genetics and Personalized Medicine, Danish Epilepsy Center Filadelfia, Dianalund, Denmark
Amplexa Genetics, Odense, Denmark
Search for more papers by this authorStephanie Schorge
Department of Clinical and Experimental Epilepsy, Institute of Neurology, University College London, London, UK
School of Pharmacy, University College London, London, UK
Search for more papers by this authorDavid Baez-Nieto
Stanley Center for Psychiatric Research, Broad Institute of Massachusetts Institute of Technology and Harvard, Cambridge, Massachusetts
Search for more papers by this authorHao-Ran Wang
Stanley Center for Psychiatric Research, Broad Institute of Massachusetts Institute of Technology and Harvard, Cambridge, Massachusetts
Search for more papers by this authorAndrew Allen
Stanley Center for Psychiatric Research, Broad Institute of Massachusetts Institute of Technology and Harvard, Cambridge, Massachusetts
Search for more papers by this authorJen Q. Pan
Stanley Center for Psychiatric Research, Broad Institute of Massachusetts Institute of Technology and Harvard, Cambridge, Massachusetts
Search for more papers by this authorHolger Lerche
Department of Neurology and Epileptology, Hertie Institute for Clinical Brain Research, University of Tübingen, Germany
Search for more papers by this authorHenrike Heyne
Stanley Center for Psychiatric Research, Broad Institute of Massachusetts Institute of Technology and Harvard, Cambridge, Massachusetts
Analytic and Translational Genetics Unit, Massachusetts General Hospital, Boston, Massachusetts
Institute for Molecular Medicine Finland, University of Helsinki, Helsinki, Finland
Search for more papers by this authorJoseph D. Symonds
Paediatric Neurosciences Research Group, Royal Hospital for Children, Glasgow, UK
School of Medicine, University of Glasgow, Glasgow, UK
Search for more papers by this authorSameer M. Zuberi
Paediatric Neurosciences Research Group, Royal Hospital for Children, Glasgow, UK
School of Medicine, University of Glasgow, Glasgow, UK
Search for more papers by this authorStephan Sanders
Department of Psychiatry, UCSF Weill Institute for Neurosciences, University of California, San Francisco, San Francisco, California
Search for more papers by this authorBeth R. Sheidley
Epilepsy Genetics Program, Division of Epilepsy and Clinical Neurophysiology, Department of Neurology, Boston Children's Hospital, Boston, Massachusetts
Search for more papers by this authorDana Craiu
Carol Davila University of Medicine, Department of Clinical Neurosciences, Pediatric Neurology Discipline, Bucharest, Romania
Alexandru Obregia Hospital, Pediatric Neurology Clinic, Bucharest, Romania
Search for more papers by this authorHeather E. Olson
Epilepsy Genetics Program, Division of Epilepsy and Clinical Neurophysiology, Department of Neurology, Boston Children's Hospital, Boston, Massachusetts
Search for more papers by this authorSarah Weckhuysen
Neurogenetics Group, Center for Molecular Neurology, VIB, Antwerp, Belgium
Laboratory of Neurogenetics, Institute Born-Bunge, University of Antwerp, Antwerp, Belgium
Department of Neurology, University Hospital Antwerp, Antwerp, Belgium
Search for more papers by this authorPeter DeJonge
Neurogenetics Group, Center for Molecular Neurology, VIB, Antwerp, Belgium
Laboratory of Neurogenetics, Institute Born-Bunge, University of Antwerp, Antwerp, Belgium
Department of Neurology, University Hospital Antwerp, Antwerp, Belgium
Search for more papers by this authorIngo Helbig
Division of Neurology, Children's Hospital of Philadelphia, Philadelphia, Pennsylvania
Epilepsy NeuroGenetics Initiative, Children's Hospital of Philadelphia, Philadelphia, Pennsylvania
Department of Biomedical and Health Informatics, Children's Hospital of Philadelphia, Philadelphia, Pennsylvania
Department of Neurology, Perelman School of Medicine, University of Pennsylvania, Philadelphia, Pennsylvania
Department of Neuropediatrics, University of Kiel, Kiel, Germany
Search for more papers by this authorHilde Van Esch
Department of Human Genetics and Center for Human Genetics, Laboratory for Genetics of Cognition, University Hospitals Leuven, Leuven, Belgium
Search for more papers by this authorTiffany Busa
Genetics Department, Timone Enfants University Hospital Center, Public Assistance–Marseille Hospitals, Marseille, France
Search for more papers by this authorMatthieu Milh
Medical Genetics and Functional Genomics, National Institute of Health and Medical Research, Mixed Unit of Research S910, Aix-Marseille University, Marseille, France
Hematology Laboratory, Le Mans Hospital Center, Le Mans, France
Search for more papers by this authorBertrand Isidor
Medical Genetics Department, Nantes University Hospital Center, Nantes, France
Search for more papers by this authorChristel Depienne
Institute of Human Genetics, Essen University Hospital, Essen, Germany
Brain and Spinal Cord Institute, National Institute of Health and Medical Research, Unit 1127, National Center for Scientific Research, Mixed Unit of Research 7225, Sorbonne Universities, Pierre and Marie Curie University, Mixed Unit of Research S 1127, Brain & Spine Institute, Paris, France
Search for more papers by this authorAnnapurna Poduri
Epilepsy Genetics Program, Division of Epilepsy and Clinical Neurophysiology, Department of Neurology, Boston Children's Hospital, Boston, Massachusetts
Harvard Medical School, Boston, Massachusetts
Search for more papers by this authorArthur J. Campbell
School of Pharmacy, University College London, London, UK
Search for more papers by this authorJordane Dimidschstein
Stanley Center for Psychiatric Research, Broad Institute of Massachusetts Institute of Technology and Harvard, Cambridge, Massachusetts
Search for more papers by this authorCorresponding Author
Rikke S. Møller
Deparment of Epilepsy Genetics and Personalized Medicine, Danish Epilepsy Center Filadelfia, Dianalund, Denmark
Institute for Regional Health Services, University of Southern Denmark, Odense, Denmark
Correspondence
Andreas Brunklaus, Fraser of Allander Neurosciences Unit, Office Block, Ground Floor, Zone 2, Royal Hospital for Children, 1345 Govan Road, Glasgow G51 4TF, UK.
Email: [email protected]
Rikke S. Møller, Head of Department of Epilepsy Genetics and Personalized Medicine, Danish Epilepsy Centre/University of Southern Denmark, Medicine Kolonivej 1, 4293, Dianalund, Denmark.
Email: [email protected]
Dennis Lal, Genomic Medicine Institute, Cleveland Clinic Lerner College of Medicine of Case Western Reserve University, US, Epilepsy Institute, Cleveland Clinic, 9500 Euclid Ave, NE5-308, Cleveland, OH 44195, USA.
Emails: [email protected]; [email protected]
Search for more papers by this authorCorresponding Author
Dennis Lal
Cologne Center for Genomics, University of Cologne, University Hospital Cologne, Cologne, Germany
Stanley Center for Psychiatric Research, Broad Institute of Massachusetts Institute of Technology and Harvard, Cambridge, Massachusetts
Analytic and Translational Genetics Unit, Massachusetts General Hospital, Boston, Massachusetts
Epilepsy Center, Neurological Institute, Cleveland Clinic, Cleveland, Ohio
Genomic Medicine Institute, Lerner Research Institute, Cleveland Clinic, Cleveland, Ohio
Andreas Brunklaus, Juanjiangmeng Du and Dennis Lal contributed equally to this work
Correspondence
Andreas Brunklaus, Fraser of Allander Neurosciences Unit, Office Block, Ground Floor, Zone 2, Royal Hospital for Children, 1345 Govan Road, Glasgow G51 4TF, UK.
Email: [email protected]
Rikke S. Møller, Head of Department of Epilepsy Genetics and Personalized Medicine, Danish Epilepsy Centre/University of Southern Denmark, Medicine Kolonivej 1, 4293, Dianalund, Denmark.
Email: [email protected]
Dennis Lal, Genomic Medicine Institute, Cleveland Clinic Lerner College of Medicine of Case Western Reserve University, US, Epilepsy Institute, Cleveland Clinic, 9500 Euclid Ave, NE5-308, Cleveland, OH 44195, USA.
Emails: [email protected]; [email protected]
Search for more papers by this authorCorresponding Author
Andreas Brunklaus
Paediatric Neurosciences Research Group, Royal Hospital for Children, Glasgow, UK
School of Medicine, University of Glasgow, Glasgow, UK
Andreas Brunklaus, Juanjiangmeng Du and Dennis Lal contributed equally to this work
Correspondence
Andreas Brunklaus, Fraser of Allander Neurosciences Unit, Office Block, Ground Floor, Zone 2, Royal Hospital for Children, 1345 Govan Road, Glasgow G51 4TF, UK.
Email: [email protected]
Rikke S. Møller, Head of Department of Epilepsy Genetics and Personalized Medicine, Danish Epilepsy Centre/University of Southern Denmark, Medicine Kolonivej 1, 4293, Dianalund, Denmark.
Email: [email protected]
Dennis Lal, Genomic Medicine Institute, Cleveland Clinic Lerner College of Medicine of Case Western Reserve University, US, Epilepsy Institute, Cleveland Clinic, 9500 Euclid Ave, NE5-308, Cleveland, OH 44195, USA.
Emails: [email protected]; [email protected]
Search for more papers by this authorJuanjiangmeng Du
Cologne Center for Genomics, University of Cologne, University Hospital Cologne, Cologne, Germany
Andreas Brunklaus, Juanjiangmeng Du and Dennis Lal contributed equally to this work
Search for more papers by this authorFelix Steckler
Paediatric Neurosciences Research Group, Royal Hospital for Children, Glasgow, UK
School of Medicine, University of Glasgow, Glasgow, UK
Search for more papers by this authorIsmael I. Ghanty
Paediatric Neurosciences Research Group, Royal Hospital for Children, Glasgow, UK
School of Medicine, University of Glasgow, Glasgow, UK
Search for more papers by this authorKatrine M. Johannesen
Deparment of Epilepsy Genetics and Personalized Medicine, Danish Epilepsy Center Filadelfia, Dianalund, Denmark
Institute for Regional Health Services, University of Southern Denmark, Odense, Denmark
Search for more papers by this authorChristina Dühring Fenger
Deparment of Epilepsy Genetics and Personalized Medicine, Danish Epilepsy Center Filadelfia, Dianalund, Denmark
Amplexa Genetics, Odense, Denmark
Search for more papers by this authorStephanie Schorge
Department of Clinical and Experimental Epilepsy, Institute of Neurology, University College London, London, UK
School of Pharmacy, University College London, London, UK
Search for more papers by this authorDavid Baez-Nieto
Stanley Center for Psychiatric Research, Broad Institute of Massachusetts Institute of Technology and Harvard, Cambridge, Massachusetts
Search for more papers by this authorHao-Ran Wang
Stanley Center for Psychiatric Research, Broad Institute of Massachusetts Institute of Technology and Harvard, Cambridge, Massachusetts
Search for more papers by this authorAndrew Allen
Stanley Center for Psychiatric Research, Broad Institute of Massachusetts Institute of Technology and Harvard, Cambridge, Massachusetts
Search for more papers by this authorJen Q. Pan
Stanley Center for Psychiatric Research, Broad Institute of Massachusetts Institute of Technology and Harvard, Cambridge, Massachusetts
Search for more papers by this authorHolger Lerche
Department of Neurology and Epileptology, Hertie Institute for Clinical Brain Research, University of Tübingen, Germany
Search for more papers by this authorHenrike Heyne
Stanley Center for Psychiatric Research, Broad Institute of Massachusetts Institute of Technology and Harvard, Cambridge, Massachusetts
Analytic and Translational Genetics Unit, Massachusetts General Hospital, Boston, Massachusetts
Institute for Molecular Medicine Finland, University of Helsinki, Helsinki, Finland
Search for more papers by this authorJoseph D. Symonds
Paediatric Neurosciences Research Group, Royal Hospital for Children, Glasgow, UK
School of Medicine, University of Glasgow, Glasgow, UK
Search for more papers by this authorSameer M. Zuberi
Paediatric Neurosciences Research Group, Royal Hospital for Children, Glasgow, UK
School of Medicine, University of Glasgow, Glasgow, UK
Search for more papers by this authorStephan Sanders
Department of Psychiatry, UCSF Weill Institute for Neurosciences, University of California, San Francisco, San Francisco, California
Search for more papers by this authorBeth R. Sheidley
Epilepsy Genetics Program, Division of Epilepsy and Clinical Neurophysiology, Department of Neurology, Boston Children's Hospital, Boston, Massachusetts
Search for more papers by this authorDana Craiu
Carol Davila University of Medicine, Department of Clinical Neurosciences, Pediatric Neurology Discipline, Bucharest, Romania
Alexandru Obregia Hospital, Pediatric Neurology Clinic, Bucharest, Romania
Search for more papers by this authorHeather E. Olson
Epilepsy Genetics Program, Division of Epilepsy and Clinical Neurophysiology, Department of Neurology, Boston Children's Hospital, Boston, Massachusetts
Search for more papers by this authorSarah Weckhuysen
Neurogenetics Group, Center for Molecular Neurology, VIB, Antwerp, Belgium
Laboratory of Neurogenetics, Institute Born-Bunge, University of Antwerp, Antwerp, Belgium
Department of Neurology, University Hospital Antwerp, Antwerp, Belgium
Search for more papers by this authorPeter DeJonge
Neurogenetics Group, Center for Molecular Neurology, VIB, Antwerp, Belgium
Laboratory of Neurogenetics, Institute Born-Bunge, University of Antwerp, Antwerp, Belgium
Department of Neurology, University Hospital Antwerp, Antwerp, Belgium
Search for more papers by this authorIngo Helbig
Division of Neurology, Children's Hospital of Philadelphia, Philadelphia, Pennsylvania
Epilepsy NeuroGenetics Initiative, Children's Hospital of Philadelphia, Philadelphia, Pennsylvania
Department of Biomedical and Health Informatics, Children's Hospital of Philadelphia, Philadelphia, Pennsylvania
Department of Neurology, Perelman School of Medicine, University of Pennsylvania, Philadelphia, Pennsylvania
Department of Neuropediatrics, University of Kiel, Kiel, Germany
Search for more papers by this authorHilde Van Esch
Department of Human Genetics and Center for Human Genetics, Laboratory for Genetics of Cognition, University Hospitals Leuven, Leuven, Belgium
Search for more papers by this authorTiffany Busa
Genetics Department, Timone Enfants University Hospital Center, Public Assistance–Marseille Hospitals, Marseille, France
Search for more papers by this authorMatthieu Milh
Medical Genetics and Functional Genomics, National Institute of Health and Medical Research, Mixed Unit of Research S910, Aix-Marseille University, Marseille, France
Hematology Laboratory, Le Mans Hospital Center, Le Mans, France
Search for more papers by this authorBertrand Isidor
Medical Genetics Department, Nantes University Hospital Center, Nantes, France
Search for more papers by this authorChristel Depienne
Institute of Human Genetics, Essen University Hospital, Essen, Germany
Brain and Spinal Cord Institute, National Institute of Health and Medical Research, Unit 1127, National Center for Scientific Research, Mixed Unit of Research 7225, Sorbonne Universities, Pierre and Marie Curie University, Mixed Unit of Research S 1127, Brain & Spine Institute, Paris, France
Search for more papers by this authorAnnapurna Poduri
Epilepsy Genetics Program, Division of Epilepsy and Clinical Neurophysiology, Department of Neurology, Boston Children's Hospital, Boston, Massachusetts
Harvard Medical School, Boston, Massachusetts
Search for more papers by this authorArthur J. Campbell
School of Pharmacy, University College London, London, UK
Search for more papers by this authorJordane Dimidschstein
Stanley Center for Psychiatric Research, Broad Institute of Massachusetts Institute of Technology and Harvard, Cambridge, Massachusetts
Search for more papers by this authorCorresponding Author
Rikke S. Møller
Deparment of Epilepsy Genetics and Personalized Medicine, Danish Epilepsy Center Filadelfia, Dianalund, Denmark
Institute for Regional Health Services, University of Southern Denmark, Odense, Denmark
Correspondence
Andreas Brunklaus, Fraser of Allander Neurosciences Unit, Office Block, Ground Floor, Zone 2, Royal Hospital for Children, 1345 Govan Road, Glasgow G51 4TF, UK.
Email: [email protected]
Rikke S. Møller, Head of Department of Epilepsy Genetics and Personalized Medicine, Danish Epilepsy Centre/University of Southern Denmark, Medicine Kolonivej 1, 4293, Dianalund, Denmark.
Email: [email protected]
Dennis Lal, Genomic Medicine Institute, Cleveland Clinic Lerner College of Medicine of Case Western Reserve University, US, Epilepsy Institute, Cleveland Clinic, 9500 Euclid Ave, NE5-308, Cleveland, OH 44195, USA.
Emails: [email protected]; [email protected]
Search for more papers by this authorCorresponding Author
Dennis Lal
Cologne Center for Genomics, University of Cologne, University Hospital Cologne, Cologne, Germany
Stanley Center for Psychiatric Research, Broad Institute of Massachusetts Institute of Technology and Harvard, Cambridge, Massachusetts
Analytic and Translational Genetics Unit, Massachusetts General Hospital, Boston, Massachusetts
Epilepsy Center, Neurological Institute, Cleveland Clinic, Cleveland, Ohio
Genomic Medicine Institute, Lerner Research Institute, Cleveland Clinic, Cleveland, Ohio
Andreas Brunklaus, Juanjiangmeng Du and Dennis Lal contributed equally to this work
Correspondence
Andreas Brunklaus, Fraser of Allander Neurosciences Unit, Office Block, Ground Floor, Zone 2, Royal Hospital for Children, 1345 Govan Road, Glasgow G51 4TF, UK.
Email: [email protected]
Rikke S. Møller, Head of Department of Epilepsy Genetics and Personalized Medicine, Danish Epilepsy Centre/University of Southern Denmark, Medicine Kolonivej 1, 4293, Dianalund, Denmark.
Email: [email protected]
Dennis Lal, Genomic Medicine Institute, Cleveland Clinic Lerner College of Medicine of Case Western Reserve University, US, Epilepsy Institute, Cleveland Clinic, 9500 Euclid Ave, NE5-308, Cleveland, OH 44195, USA.
Emails: [email protected]; [email protected]
Search for more papers by this authorFunding information
J.Du was supported by the Koeln Fortune (grant number 241/2017).
Abstract
Objective
Voltage-gated sodium channels (SCNs) share similar amino acid sequence, structure, and function. Genetic variants in the four human brain-expressed SCN genes SCN1A/2A/3A/8A have been associated with heterogeneous epilepsy phenotypes and neurodevelopmental disorders. To better understand the biology of seizure susceptibility in SCN-related epilepsies, our aim was to determine similarities and differences between sodium channel disorders, allowing us to develop a broader perspective on precision treatment than on an individual gene level alone.
Methods
We analyzed genotype-phenotype correlations in large SCN-patient cohorts and applied variant constraint analysis to identify severe sodium channel disease. We examined temporal patterns of human SCN expression and correlated functional data from in vitro studies with clinical phenotypes across different sodium channel disorders.
Results
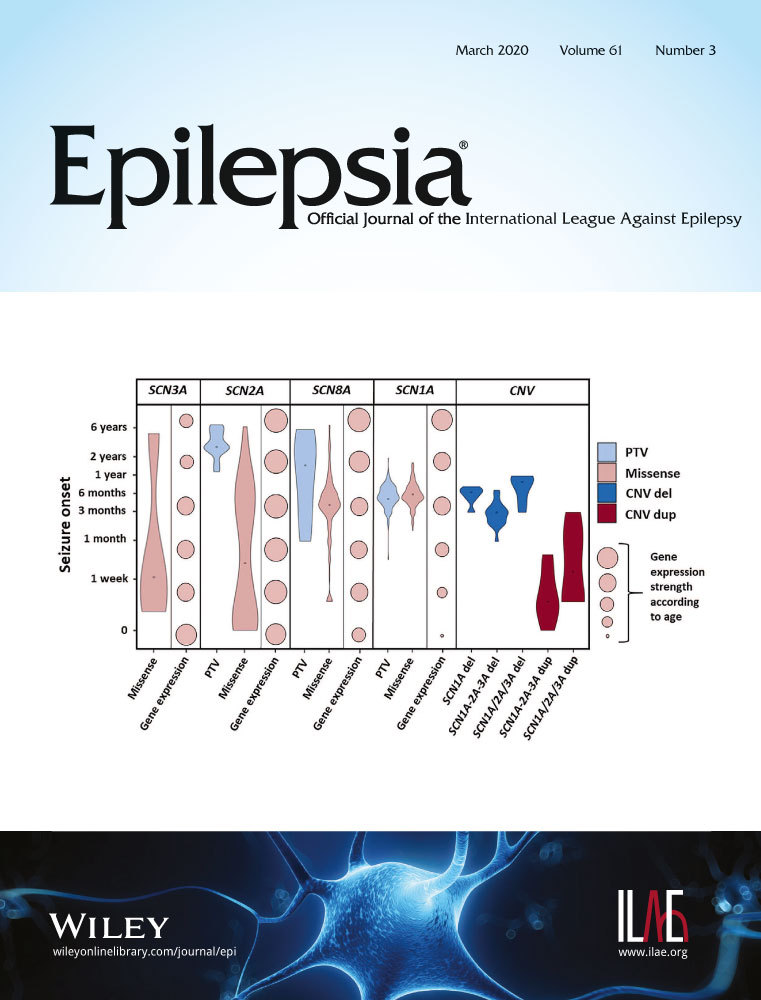
Comparing 865 epilepsy patients (504 SCN1A, 140 SCN2A, 171 SCN8A, four SCN3A, 46 copy number variation [CNV] cases) and analysis of 114 functional studies allowed us to identify common patterns of presentation. All four epilepsy-associated SCN genes demonstrated significant constraint in both protein truncating and missense variation when compared to other SCN genes. We observed that age at seizure onset is related to SCN gene expression over time. Individuals with gain-of-function SCN2A/3A/8A missense variants or CNV duplications share similar characteristics, most frequently present with early onset epilepsy (<3 months), and demonstrate good response to sodium channel blockers (SCBs). Direct comparison of corresponding SCN variants across different SCN subtypes illustrates that the functional effects of variants in corresponding channel locations are similar; however, their clinical manifestation differs, depending on their role in different types of neurons in which they are expressed.
Significance
Variant function and location within one channel can serve as a surrogate for variant effects across related sodium channels. Taking a broader view on precision treatment suggests that in those patients with a suspected underlying genetic epilepsy presenting with neonatal or early onset seizures (<3 months), SCBs should be considered.
CONFLICT OF INTEREST
None of the authors has any conflict of interest to disclose. We confirm that we have read the Journal's position on issues involved in ethical publication and affirm that this report is consistent with those guidelines.
Supporting Information
| Filename | Description |
|---|---|
| epi16438-sup-0001-TableS1.xlsxapplication/excel, 47.6 KB | |
| epi16438-sup-0002-TableS2.docxWord document, 87.8 KB | |
| epi16438-sup-0003-TableS3.docxWord document, 267 KB | |
| epi16438-sup-0004-TableS4.docxWord document, 18.6 KB | |
| epi16438-sup-0005-TableS5.docxWord document, 20.3 KB |
Please note: The publisher is not responsible for the content or functionality of any supporting information supplied by the authors. Any queries (other than missing content) should be directed to the corresponding author for the article.
REFERENCES
- 1Lal D, May P, Samocha KE, et al. Gene family information facilitates variant interpretation and identification of disease-associated genes. bioRxiv. 2017; 159780. https://doi.org/10.1101/159780
- 2Zuberi SM, Brunklaus A, Birch R, Reavey E, Duncan J, Forbes GH. Genotype-phenotype associations in SCN1A-related epilepsies. Neurology. 2011; 76: 594–600.
- 3Claes LR, Deprez L, Suls A, et al. The SCN1A variant database: a novel research and diagnostic tool. Hum Mutat. 2009; 30: E904–20.
- 4Escayg A, MacDonald BT, Meisler MH, et al. Mutations of SCN1A, encoding a neuronal sodium channel, in two families with GEFS+2. Nat Genet. 2000; 24: 343–5.
- 5Marini C, Scheffer IE, Nabbout R, et al. SCN1A duplications and deletions detected in Dravet syndrome: implications for molecular diagnosis. Epilepsia. 2009; 50: 1670–8.
- 6Davidsson J, Collin A, Olsson ME, Lundgren J, Soller M. Deletion of the SCN gene cluster on 2q24.4 is associated with severe epilepsy: an array-based genotype–phenotype correlation and a comprehensive review of previously published cases. Epilepsy Res. 2008; 81: 69–79.
- 7Wang J, Kurahashi H, Ishii A, et al. Microchromosomal deletions involving SCN1A and adjacent genes in severe myoclonic epilepsy in infancy. Epilepsia. 2008; 49: 1528–34.
- 8Brunklaus A, Ellis R, Reavey E, Forbes GH, Zuberi SM. Prognostic, clinical and demographic features in SCN1A mutation-positive Dravet syndrome. Brain. 2012; 135: 2329–36.
- 9Dravet C. Les epilepsies graves de l'enfant. Vie Med. 1978; 8: 543–8.
- 10Mantegazza M, Cestèle S. Pathophysiological mechanisms of migraine and epilepsy: similarities and differences. Neurosci Lett. 2018; 667: 92–102.
- 11Ogiwara I, Ito K, Sawaishi Y, et al. De novo mutations of voltage-gated sodium channel αII gene SCN2A in intractable epilepsies. Neurology. 2009; 73: 1046–53.
- 12Heron SE, Crossland KM, Andermann E, et al. Sodium-channel defects in benign familial neonatal-infantile seizures. Lancet. 2002; 360: 851–2.
- 13Nakamura K, Kato M, Osaka H, et al. Clinical spectrum of SCN2A mutations expanding to Ohtahara syndrome. Neurology. 2013; 81: 992–8.
- 14Ben-Shalom R, Keeshen CM, Berrios KN, An JY, Sanders SJ, Bender KJ. Opposing effects on NaV 1.2 function underlie differences between SCN2A variants observed in individuals with autism spectrum disorder or infantile seizures. Biol Psychiatry. 2017; 82: 224–32.
- 15Wolff M, Johannesen KM, Hedrich UBS, Masnada S, Rubboli G, Gardella E, et al. Genetic and phenotypic heterogeneity suggest therapeutic implications in SCN2A-related disorders. Brain. 2017; 140: 1316–36.
- 16Lauxmann S, Verbeek NE, Liu Y, et al. Relationship of electrophysiological dysfunction and clinical severity in SCN2A-related epilepsies. Hum Mutat. 2018; 39: 1942–56.
- 17Begemann A, Acuña MA, Zweier M, Vincent M, Steindl K, Bachmann-Gagescu R, et al. Further corroboration of distinct functional features in SCN2A variants causing intellectual disability or epileptic phenotypes. Mol Med. 2019; 25: 6. https://doi.org/10.1186/s10020-019-0073-6
- 18Berecki G, Howell KB, Deerasooriya YH, et al. Dynamic action potential clamp predicts functional separation in mild familial and severe de novo forms of SCN2A epilepsy. Proc Natl Acad Sci U S A. 2018; 115: E5516–25.
- 19Larsen J, Carvill GL, Gardella E, et al. The phenotypic spectrum of SCN8A encephalopathy. Neurology. 2015; 84: 480–9.
- 20Meisler MH, Helman G, Hammer MF, et al. SCN8A encephalopathy: research progress and prospects. Epilepsia. 2016; 57: 1027–35.
- 21Veeramah KR, O'Brien JE, Meisler MH, et al. De novo pathogenic SCN8A mutation identified by whole-genome sequencing of a family quartet affected by infantile epileptic encephalopathy and SUDEP. Am J Hum Genet. 2012; 90: 502–10.
- 22Gardella E, Marini C, Trivisano M, et al. The phenotype of SCN8A developmental and epileptic encephalopathy. Neurology. 2018; 91: e1112–24.
- 23Gardella E, Becker F, Møller RS, et al. Benign infantile seizures and paroxysmal dyskinesia caused by an SCN8A mutation. Ann Neurol. 2016; 79: 428–36.
- 24Wagnon JL, Barker BS, Ottolini M, Park Y, Volkheimer A, Valdez P, et al. Loss-of-function variants of SCN8A in intellectual disability without seizures. Neurol Genet. 2017; 3: 1–5.
- 25Wagnon JL, Barker BS, Hounshell JA, Haaxma CA, Shealy A, Moss T, et al. Pathogenic mechanism of recurrent mutations of SCN8A in epileptic encephalopathy. Ann Clin Transl Neurol. 2015; 3: 114–23.
- 26Liu Y, Schubert J, Sonnenberg L, et al. Neuronal mechanisms of mutations in SCN8A causing epilepsy or intellectual disability. Brain. 2019; 142: 376–90.
- 27Lamar T, Vanoye CG, Calhoun J, et al. SCN3A deficiency associated with increased seizure susceptibility. Neurobiol Dis. 2017; 102: 38–48.
- 28Zaman T, Helbig I, Božović IB, et al. Mutations in SCN3A cause early infantile epileptic encephalopathy. Ann Neurol. 2018; 83: 703–17.
- 29Møller RS, Larsen LHG, Johannesen KM, et al. Gene panel testing in epileptic encephalopathies and familial epilepsies. Mol Syndromol. 2016; 7: 210–9.
- 30Gardella E, Møller R. Phenotypic and genetic spectrum of SCN8A-related disorders, treatment options, and outcomes. Epilepsia. 2019; 60(Suppl 3): S77–85.
- 31Johannesen KM, Gardella E, Encinas AC, et al. The spectrum of intermediate SCN8A-related epilepsy. Epilepsia. 2019; 60: 830–44.
- 32Depienne C, Trouillard O, Saint-Martin C, et al. Spectrum of SCN1A gene mutations associated with Dravet syndrome: analysis of 333 patients. J Med Genet. 2009; 46: 183–91.
- 33Firth HV, Richards SM, Bevan AP, et al. DECIPHER: database of chromosomal imbalance and phenotype in humans using ensembl resources. Am J Hum Genet. 2009; 84: 524–33.
- 34Lek M, Karczewski KJ, Minikel EV, et al. Analysis of protein-coding genetic variation in 60,706 humans. Nature. 2016; 536: 285–91.
- 35Catterall WA, Goldin AL, Waxman SG. International Union of Pharmacology. XLVII. Nomenclature and structure-function relationships of voltage-gated sodium channels. Pharmacol Rev. 2005; 57: 397–409.
- 36Cetica V, Chiari S, Mei D, et al. Clinical and genetic factors predicting Dravet syndrome in infants with SCN1A mutations. Neurology. 2017; 88: 1037–44.
- 37Sadleir LG, Mountier EI, Gill D, et al. Not all SCN1A epileptic encephalopathies are Dravet syndrome. Neurology. 2017; 89: 1035–42.
- 38Berecki G, Bryson A, Terhag J, et al. SCN1A gain of function in early infantile encephalopathy. Ann Neurol. 2019; 85: 514–25.
- 39Beck VC, Hull JM, Isom LL. Beyond Dravet syndrome: characterization of a novel, more severe SCN1A-linked epileptic encephalopathy. Epilepsy Curr. 2019; 19: 266–8.
- 40Sanders SJ, Campbell AJ, Cottrell JR, et al. Progress in understanding and treating SCN2A-mediated disorders. Trends Neurosci. 2018; 41: 442–56.
- 41Thuresson A-C, Van Buggenhout G, Sheth F, et al. Whole gene duplication of SCN2A and SCN3A is associated with neonatal seizures and a normal intellectual development. Clin Genet. 2017; 91: 106–10.
- 42Catterall WA. Voltage-gated sodium channels at 60: structure, function and pathophysiology. J Physiol. 2012; 590: 2577–89.
- 43Brunklaus A, Ellis R, Reavey E, Semsarian C, Zuberi SM. Genotype phenotype associations across the voltage-gated sodium channel family. J Med Genet. 2014; 51: 650–8.
- 44Holland KD, Bouley TM, Horn PS. Location: a surrogate for personalized treatment of sodium channelopathies. Ann Neurol. 2018; 84: 1–9.
- 45Cheah CS, Westenbroek RE, Roden WH, et al. Correlations in timing of sodium channel expression, epilepsy, and sudden death in Dravet syndrome. Channels. 2013; 7: 468–72.
- 46Biella G, Di Febo F, Goffredo D, et al. Differentiating embryonic stem–derived neural stem cells show a maturation-dependent pattern of voltage-gated sodium current expression and graded action potentials. Neuroscience. 2007; 149: 38–52.
- 47Liu Y, Lopez-Santiago LF, Yuan Y, et al. Dravet syndrome patient-derived neurons suggest a novel epilepsy mechanism. Ann Neurol. 2013; 74: 128–39.
- 48Escayg A, Goldin AL. Sodium channel SCN1A and epilepsy: mutations and mechanisms. Epilepsia. 2010; 51: 1650–8.
- 49Liao Y, Deprez L, Maljevic S, et al. Molecular correlates of age-dependent seizures in an inherited neonatal-infantile epilepsy. Brain. 2010; 133: 1403–14.
- 50Liao Y, Anttonen A-K, Liukkonen E, et al. SCN2A mutation associated with neonatal epilepsy, late-onset episodic ataxia, myoclonus, and pain. Neurology. 2010; 75: 1454–8.
- 51Van Wart A, Matthews G. Impaired firing and cell-specific compensation in neurons lacking Nav1.6 sodium channels. J Neurosci. 2006; 26: 7172–80.
- 52Guerrini R, Dravet C, Genton P, Belmonte A, Kaminska A, Dulac O. Lamotrigine and seizure aggravation in severe myoclonic epilepsy. Epilepsia. 1998; 39: 508–12.
- 53Møller RS, Johannesen KM. Precision medicine: SCN8A encephalopathy treated with sodium channel blockers. Neurotherapeutics. 2016; 13: 190–1.
- 54Symonds JD, Zuberi SM, Stewart K, et al. Incidence and phenotypes of childhood-onset genetic epilepsies: a prospective population-based national cohort. Brain. 2019; 142: 2303–18.
- 55Olson HE, Kelly M, LaCoursiere CM, et al. Genetics and genotype-phenotype correlations in early onset epileptic encephalopathy with burst suppression. Ann Neurol. 2017; 81: 419–29.
- 56Cestele S, Schiavon E, Rusconi R, Franceschetti S, Mantegazza M. Nonfunctional NaV1.1 familial hemiplegic migraine mutant transformed into gain of function by partial rescue of folding defects. Proc Natl Acad Sci. 2013; 110: 17546–51.
- 57Denis J, Villeneuve N, Cacciagli P, Mignon-Ravix C, Lacoste C, Lefranc J, et al. Clinical study of 19 patients with SCN8A-related epilepsy: two modes of onset regarding EEG and seizures. Epilepsia. 2019; 60: 845–56.
- 58Kahlig KM, Rhodes TH, Pusch M, Freilinger T, Pereira-Monteiro JM, Ferrari MD, et al. Divergent sodium channel defects in familial hemiplegic migraine. Proc Natl Acad Sci. 2008; 105: 9799–804.
- 59Lossin C, Rhodes TH, Desai RR, Vanoye CG, Wang D, Carniciu S, et al. Epilepsy-associated dysfunction in the voltage-gated neuronal sodium channel SCN1A. J Neurosci. 2003; 23: 11289–95.
- 60Rhodes TH, Vanoye CG, Ohmori I, Ogiwara I, Yamakawa K, George AL. Sodium channel dysfunction in intractable childhood epilepsy with generalized tonic-clonic seizures. J Physiol. 2005; 569: 433–45.
- 61Volkers L, Kahlig KM, Verbeek NE, Das JHG, van Kempen MJA, Stroink H, et al. Nav1.1 dysfunction in genetic epilepsy with febrile seizures-plus or Dravet syndrome. Eur J Neurosci. 2011; 34: 1268–75.
- 62Wengert ER, Tronhjem CE, Wagnon JL, Johannesen KM, Petit H, Krey I, et al. Biallelic inherited SCN8A variants, a rare cause of SCN8A-related developmental and epileptic encephalopathy. Epilepsia. 2019; 60: 2277–85.