Clinical features and survival of patients with indolent systemic mastocytosis defined by the updated WHO classification
Jakub Trizuljak
Department of Hematology and Oncology, University Hospital, CEITEC Masaryk University, Brno, Czech Republic
Search for more papers by this authorWolfgang R. Sperr
Department of Internal Medicine I, Division of Hematology and Hemostaseology, Ludwig Boltzmann Institute for Hematology and Oncology, Medical University of Vienna, Vienna, Austria
Search for more papers by this authorLucie Nekvindová
Institute of Biostatistics and Analyses, Ltd., Spinoff Company of Masaryk University, Brno, Czech Republic
Search for more papers by this authorHanneke O. Elberink
Department of Allergology, University Medical Center Groningen, University of Groningen, Groningen, the Netherlands
Search for more papers by this authorKaroline V. Gleixner
Department of Internal Medicine I, Division of Hematology and Hemostaseology, Ludwig Boltzmann Institute for Hematology and Oncology, Medical University of Vienna, Vienna, Austria
Search for more papers by this authorAleksandra Gorska
Department of Allergology, Medical University of Gdańsk, Gdańsk, Poland
Search for more papers by this authorMagdalena Lange
Department of Dermatology, Venereology and Allergology, Medical University of Gdańsk, Gdańsk, Poland
Search for more papers by this authorKarin Hartmann
Division of Allergy, Department of Dermatology, University of Basel, Basel, Switzerland
Department of Dermatology, University of Cologne, Cologne, Germany
Search for more papers by this authorAnja Illerhaus
Department of Dermatology, University of Cologne, Cologne, Germany
Search for more papers by this authorMassimiliano Bonifacio
Department of Medicine, Section of Hematology, University of Verona, Verona, Italy
Search for more papers by this authorCecelia Perkins
Division of Hematology, Department of Medicine, Stanford University, School of Medicine/Stanford Cancer Institute, Stanford, CA, USA
Search for more papers by this authorChiara Elena
Division of Hematology, Fondazione IRCCS Policlinico San Matteo & University of Pavia, Pavia, Italy
Search for more papers by this authorLuca Malcovati
Division of Hematology, Fondazione IRCCS Policlinico San Matteo & University of Pavia, Pavia, Italy
Search for more papers by this authorAnna B. Fortina
Pediatric Dermatology Unit, Department of Medicine, University of Padova, Padova, Italy
Search for more papers by this authorKhalid Shoumariyeh
Department of Hematology, Oncology and Stem Cell Transplantation, Medical Center, Faculty of Medicine, University of Freiburg, Freiburg, Germany
Search for more papers by this authorMohamad Jawhar
Hämatologie und Onkologie, III. Medizinische Klinik, Universitätsmedizin Mannheim, Universität Heidelberg, Mannheim, Germany
Search for more papers by this authorRoberta Zanotti
Department of Medicine, Section of Hematology, University of Verona, Verona, Italy
Search for more papers by this authorPatrizia Bonadonna
Allergy Unit, Verona University Hospital, Verona, Italy
Search for more papers by this authorFrancesca Caroppo
Pediatric Dermatology Unit, Department of Medicine, University of Padova, Padova, Italy
Search for more papers by this authorAlexander Zink
Department of Dermatology and Allergy Biederstein, School of Medicine, Technical University of Munich, Munich, Germany
Search for more papers by this authorMassimo Triggiani
Division of Allergy and Clinical Immunology, University of Salerno, Salerno, Italy
Search for more papers by this authorRoberta Parente
Division of Allergy and Clinical Immunology, University of Salerno, Salerno, Italy
Search for more papers by this authorNikolas von Bubnoff
Department of Hematology, Oncology and Stem Cell Transplantation, Medical Center, Faculty of Medicine, University of Freiburg, Freiburg, Germany
Search for more papers by this authorAkif S. Yavuz
Division of Hematology, Istanbul Medical School, University of Istanbul, Istanbul, Turkey
Search for more papers by this authorHans Hägglund
Department of Medical Sciences, Uppsala University, Section of Hematology, Uppsala University Hospital, Uppsala, Sweden
Search for more papers by this authorMattias Mattsson
Department of Medical Sciences, Uppsala University, Section of Hematology, Uppsala University Hospital, Uppsala, Sweden
Search for more papers by this authorJens Panse
Department of Oncology, Haematology, Haemostaseology and Stem Cell Transplantation, University Hospital RWTH Aachen, Aachen, Germany
Search for more papers by this authorNadja Jäkel
Department of Internal Medicine IV, University Hospital Halle, Halle, Germany
Search for more papers by this authorAlex Kilbertus
Department of Dermatology and Venereology, Kepler University Hospital, Johannes Kepler University, Linz, Austria
Search for more papers by this authorOlivier Hermine
Departement of Hematology, Centre National de Référence des Mastocytoses, Imagine Institute, INSERM U1123, Université Paris Descartes, Sorbonne, Paris Cité, Hôpital Necker, Assistance Publique des Hôpitaux de Paris (APHP), Paris, France
Search for more papers by this authorMichel Arock
Laboratory of Hematology, Pitié-Salpêtrière Hospital, Paris, France
Search for more papers by this authorDavid Fuchs
Department of Internal Medicine 3, Hematology and Oncology, Kepler University Hospital, Johannes Kepler University, Linz, Austria
Search for more papers by this authorVito Sabato
Faculty of Medicine and Health Sciences, Department of Immunology-Allergology-Rheumatology, University of Antwerp and Antwerp University Hospital, Antwerp, Belgium
Search for more papers by this authorKnut Brockow
Department of Dermatology and Allergy Biederstein, School of Medicine, Technical University of Munich, Munich, Germany
Search for more papers by this authorAgnes Bretterklieber
Department of Dermatology and Venereology, Medical University of Graz, Graz, Austria
Search for more papers by this authorMarek Niedoszytko
Department of Allergology, Medical University of Gdańsk, Gdańsk, Poland
Search for more papers by this authorBjörn van Anrooij
Department of Allergology, University Medical Center Groningen, University of Groningen, Groningen, the Netherlands
Search for more papers by this authorAndreas Reiter
Hämatologie und Onkologie, III. Medizinische Klinik, Universitätsmedizin Mannheim, Universität Heidelberg, Mannheim, Germany
Search for more papers by this authorJason Gotlib
Division of Hematology, Department of Medicine, Stanford University, School of Medicine/Stanford Cancer Institute, Stanford, CA, USA
Search for more papers by this authorHanneke C. Kluin-Nelemans
Department of Hematology, University Medical Center Groningen, University of Groningen, Groningen, the Netherlands
Search for more papers by this authorJiri Mayer
Department of Hematology and Oncology, University Hospital, CEITEC Masaryk University, Brno, Czech Republic
Search for more papers by this authorCorresponding Author
Michael Doubek
Department of Hematology and Oncology, University Hospital, CEITEC Masaryk University, Brno, Czech Republic
Correspondence
Michael Doubek, University Hospital Brno and Central European Institute of Technology of Masaryk University Brno, Jihlavska 20, 62500 Brno, Czech Republic.
Email: [email protected]
Search for more papers by this authorPeter Valent
Department of Internal Medicine I, Division of Hematology and Hemostaseology, Ludwig Boltzmann Institute for Hematology and Oncology, Medical University of Vienna, Vienna, Austria
Search for more papers by this authorJakub Trizuljak
Department of Hematology and Oncology, University Hospital, CEITEC Masaryk University, Brno, Czech Republic
Search for more papers by this authorWolfgang R. Sperr
Department of Internal Medicine I, Division of Hematology and Hemostaseology, Ludwig Boltzmann Institute for Hematology and Oncology, Medical University of Vienna, Vienna, Austria
Search for more papers by this authorLucie Nekvindová
Institute of Biostatistics and Analyses, Ltd., Spinoff Company of Masaryk University, Brno, Czech Republic
Search for more papers by this authorHanneke O. Elberink
Department of Allergology, University Medical Center Groningen, University of Groningen, Groningen, the Netherlands
Search for more papers by this authorKaroline V. Gleixner
Department of Internal Medicine I, Division of Hematology and Hemostaseology, Ludwig Boltzmann Institute for Hematology and Oncology, Medical University of Vienna, Vienna, Austria
Search for more papers by this authorAleksandra Gorska
Department of Allergology, Medical University of Gdańsk, Gdańsk, Poland
Search for more papers by this authorMagdalena Lange
Department of Dermatology, Venereology and Allergology, Medical University of Gdańsk, Gdańsk, Poland
Search for more papers by this authorKarin Hartmann
Division of Allergy, Department of Dermatology, University of Basel, Basel, Switzerland
Department of Dermatology, University of Cologne, Cologne, Germany
Search for more papers by this authorAnja Illerhaus
Department of Dermatology, University of Cologne, Cologne, Germany
Search for more papers by this authorMassimiliano Bonifacio
Department of Medicine, Section of Hematology, University of Verona, Verona, Italy
Search for more papers by this authorCecelia Perkins
Division of Hematology, Department of Medicine, Stanford University, School of Medicine/Stanford Cancer Institute, Stanford, CA, USA
Search for more papers by this authorChiara Elena
Division of Hematology, Fondazione IRCCS Policlinico San Matteo & University of Pavia, Pavia, Italy
Search for more papers by this authorLuca Malcovati
Division of Hematology, Fondazione IRCCS Policlinico San Matteo & University of Pavia, Pavia, Italy
Search for more papers by this authorAnna B. Fortina
Pediatric Dermatology Unit, Department of Medicine, University of Padova, Padova, Italy
Search for more papers by this authorKhalid Shoumariyeh
Department of Hematology, Oncology and Stem Cell Transplantation, Medical Center, Faculty of Medicine, University of Freiburg, Freiburg, Germany
Search for more papers by this authorMohamad Jawhar
Hämatologie und Onkologie, III. Medizinische Klinik, Universitätsmedizin Mannheim, Universität Heidelberg, Mannheim, Germany
Search for more papers by this authorRoberta Zanotti
Department of Medicine, Section of Hematology, University of Verona, Verona, Italy
Search for more papers by this authorPatrizia Bonadonna
Allergy Unit, Verona University Hospital, Verona, Italy
Search for more papers by this authorFrancesca Caroppo
Pediatric Dermatology Unit, Department of Medicine, University of Padova, Padova, Italy
Search for more papers by this authorAlexander Zink
Department of Dermatology and Allergy Biederstein, School of Medicine, Technical University of Munich, Munich, Germany
Search for more papers by this authorMassimo Triggiani
Division of Allergy and Clinical Immunology, University of Salerno, Salerno, Italy
Search for more papers by this authorRoberta Parente
Division of Allergy and Clinical Immunology, University of Salerno, Salerno, Italy
Search for more papers by this authorNikolas von Bubnoff
Department of Hematology, Oncology and Stem Cell Transplantation, Medical Center, Faculty of Medicine, University of Freiburg, Freiburg, Germany
Search for more papers by this authorAkif S. Yavuz
Division of Hematology, Istanbul Medical School, University of Istanbul, Istanbul, Turkey
Search for more papers by this authorHans Hägglund
Department of Medical Sciences, Uppsala University, Section of Hematology, Uppsala University Hospital, Uppsala, Sweden
Search for more papers by this authorMattias Mattsson
Department of Medical Sciences, Uppsala University, Section of Hematology, Uppsala University Hospital, Uppsala, Sweden
Search for more papers by this authorJens Panse
Department of Oncology, Haematology, Haemostaseology and Stem Cell Transplantation, University Hospital RWTH Aachen, Aachen, Germany
Search for more papers by this authorNadja Jäkel
Department of Internal Medicine IV, University Hospital Halle, Halle, Germany
Search for more papers by this authorAlex Kilbertus
Department of Dermatology and Venereology, Kepler University Hospital, Johannes Kepler University, Linz, Austria
Search for more papers by this authorOlivier Hermine
Departement of Hematology, Centre National de Référence des Mastocytoses, Imagine Institute, INSERM U1123, Université Paris Descartes, Sorbonne, Paris Cité, Hôpital Necker, Assistance Publique des Hôpitaux de Paris (APHP), Paris, France
Search for more papers by this authorMichel Arock
Laboratory of Hematology, Pitié-Salpêtrière Hospital, Paris, France
Search for more papers by this authorDavid Fuchs
Department of Internal Medicine 3, Hematology and Oncology, Kepler University Hospital, Johannes Kepler University, Linz, Austria
Search for more papers by this authorVito Sabato
Faculty of Medicine and Health Sciences, Department of Immunology-Allergology-Rheumatology, University of Antwerp and Antwerp University Hospital, Antwerp, Belgium
Search for more papers by this authorKnut Brockow
Department of Dermatology and Allergy Biederstein, School of Medicine, Technical University of Munich, Munich, Germany
Search for more papers by this authorAgnes Bretterklieber
Department of Dermatology and Venereology, Medical University of Graz, Graz, Austria
Search for more papers by this authorMarek Niedoszytko
Department of Allergology, Medical University of Gdańsk, Gdańsk, Poland
Search for more papers by this authorBjörn van Anrooij
Department of Allergology, University Medical Center Groningen, University of Groningen, Groningen, the Netherlands
Search for more papers by this authorAndreas Reiter
Hämatologie und Onkologie, III. Medizinische Klinik, Universitätsmedizin Mannheim, Universität Heidelberg, Mannheim, Germany
Search for more papers by this authorJason Gotlib
Division of Hematology, Department of Medicine, Stanford University, School of Medicine/Stanford Cancer Institute, Stanford, CA, USA
Search for more papers by this authorHanneke C. Kluin-Nelemans
Department of Hematology, University Medical Center Groningen, University of Groningen, Groningen, the Netherlands
Search for more papers by this authorJiri Mayer
Department of Hematology and Oncology, University Hospital, CEITEC Masaryk University, Brno, Czech Republic
Search for more papers by this authorCorresponding Author
Michael Doubek
Department of Hematology and Oncology, University Hospital, CEITEC Masaryk University, Brno, Czech Republic
Correspondence
Michael Doubek, University Hospital Brno and Central European Institute of Technology of Masaryk University Brno, Jihlavska 20, 62500 Brno, Czech Republic.
Email: [email protected]
Search for more papers by this authorPeter Valent
Department of Internal Medicine I, Division of Hematology and Hemostaseology, Ludwig Boltzmann Institute for Hematology and Oncology, Medical University of Vienna, Vienna, Austria
Search for more papers by this authorFunding information
Supported by the project CEITEC 2020 [LQ1601] from the Ministry of Education, Youth and Sports of the Czech Republic under National Sustainability, TA CR Center of Competence project (TE02000058), MH CR – DRO (FNBr, 65269705), by Deutsche Forschungsgemeinschaft (DFG, RA 2838 to AI), by the Koeln Fortune Program, Faculty of Medicine, University of Cologne (216/2016 to AI) and by the Austrian Science Fund (FWF) grant SFB F4704-B20. Also supported by the Czech Leukemia Study Group for Life (CELL).
Abstract
Background
In indolent systemic mastocytosis (ISM), several risk factors of disease progression have been identified. Previous studies, performed with limited patient numbers, have also shown that the clinical course in ISM is stable and comparable to that of cutaneous mastocytosis (CM). The aim of this project was to compare the prognosis of patients with ISM with that of patients with CM.
Methods
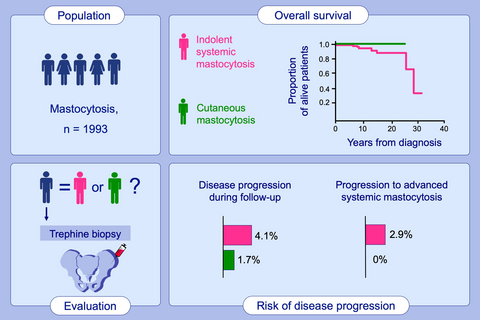
We employed a dataset of 1993 patients from the registry of the European Competence Network on Mastocytosis (ECNM) to compare outcomes of ISM and CM.
Results
We found that overall survival (OS) is worse in ISM compared to CM. Moreover, in patients with typical ISM, bone marrow mastocytosis (BMM), and smoldering SM (SSM), 4.1% of disease progressions have been observed (4.9% of progressions in typical ISM group, 1.7% in BMM, and 9.4% in SSM). Progressions to advanced SM were observed in 2.9% of these patients. In contrast, six patients with CM (1.7%) converted to ISM and no definitive progression to advanced SM was found. No significant differences in OS and event-free survival (EFS) were found when comparing ISM, BMM, and SSM. Higher risk of both progression and death was significantly associated with male gender, worse performance status, and organomegaly.
Conclusion
Our data confirm the clinical impact of the WHO classification that separates ISM from CM and from other SM variants.
Graphical Abstract
CONFLICT OF INTEREST
WRS received honoraria from Novartis, Pfizer, AbbVie, Daiichi Sankyo, Amgen, Thermo Fisher, Diciphera, Incyte, Celgene, Jazz and travel grants from Pfizer and Roche. HOE received honoraria from ALK-Abelló, Chiesi, MEDA Pharma, Novartis, Blueprint. KVG received honoraria from Novartis, Roche, BMS, Sanofi, Incyte and travel grants from Roche and AbbVie. ML received honoraria from Novartis. KH received honoraria from Novartis, ALK, Blueprint, Deciphera and research funding from Euroimmun. MB received honoraria from Amgen, Incyte, Pfizer and research funding from Novartis. CE received honoraria from Novartis and Pfizer. MJ received honoraria from Novartis, Blueprint, Deciphera. RZ is consultant Deciphera and Novartis. AZ received honoraria or participated in trials from AbbVie, Almirall, Beiersdorf Dermo Medical, Bencard Allergie, BMS, Celgene, Eli Lilly, GSK, Janssen-Cilag, Miltenyi Biotec, Novartis, Sanofi-Aventis, Takeda Pharma. MT received honoraria from Deciphera, Blueprint, Novartis. NB received honoraria from Astra Zeneca, Amgen, Novartis and BMS and research funding from Novartis. JP received honoraria from Alexion, BMS, Boehringer Ingelheim, Grünenthal, MSD, Novartis, Pfizer, Chugai. DF received honoraria from Novartis, Pfizer, Roche travel grants from Roche. VS received honoraria from Novartis, Termofisher, Shire, Stallergens. KB received honoraria from Novartis, Phadia (Thermo Fisher), Meda, BioMarin Pharmaceutical Inc outside. BA received honoraria from Novartis. AR received honoraria from Novartis, BMS, Deciphera, Blueprint, Baxalta/Shire and research funding from Novartis. JG received honoraria from Blueprint, Deciphera, Gilead, Incyte, Novartis and research funding from Blueprint, Celgene, CTI BioPharma, Deciphera, Gilead, Incyte, Pharmacyclics, Promedior, Seattle Genetics. JM received honoraria from for Novartis, Gilead, BMS. MD received honoraria from for Roche, AbbVie, Novartis, Gilead, AOP Pharmaceuticals, Janssen-Cilag. PV has received honoraria from Novartis, Pfizer, Deciphera, Incyte, Blueprint, Celgene and research funds from Pfizer, Incyte, Celgene. JT, LN, AG, AI, CP, LM, ABF, FC, KS, RP, MN, PB, ASY, HH, MM, NJ, AK, OH, MA, AB, HCKN have nothing to disclose.
Supporting Information
| Filename | Description |
|---|---|
| all14248-sup-0001-Supinfo.docxWord document, 522.3 KB | Supplementary Material |
Please note: The publisher is not responsible for the content or functionality of any supporting information supplied by the authors. Any queries (other than missing content) should be directed to the corresponding author for the article.
REFERENCES
- 1Horny H-P, Akin C, Arber DA, et al. Mastocytosis. In: SH Swerdlow, E Campo, NL Harris, ES Jaffe, SA Pileri, H Stein, J Thiele, DA Arber, RP Hasserjian, MM LeBeau, A Orazi, R Siebert, eds. WHO classification of tumours of haematopoietic and lymphoid tissues. Lyon: IARC Press; 2017: 61-70.
- 2Valent P, Akin C, Metcalfe DD. Mastocytosis: 2016 updated WHO classification and novel emerging treatment concepts. Blood. 2017; 129(11): 1420-1427.
- 3Valent P, Horny H-P, Escribano L, et al. Diagnostic criteria and classification of mastocytosis: a consensus proposal. Leuk Res. 2001; 25: 603-605.
- 4Valent P, Akin C, Hartmann K, et al. Advances in the classification and treatment of mastocytosis: current status and outlook toward the future. Cancer Res. 2017; 77(6): 1261-1270.
- 5Sperr WR, Valent P. Diagnosis, progression patterns and prognostication in mastocytosis. Expert Rev Hematol. 2012; 5: 261-274.
- 6Lim K-H, Tefferi A, Lasho TL, et al. Systemic mastocytosis in 342 consecutive adults: survival studies and prognostic factors. Blood. 2009; 113(23): 5727-5736.
- 7Arock M, Valent P. Pathogenesis, classification and treatment of mastocytosis: state of the art in 2010 and future perspectives. Expert Rev Hematol. 2010; 3: 497-516.
- 8Sperr WR, Escribano L, Jordan J-H, et al. Morphologic properties of neoplastic mast cells: delineation of stages of maturation and implication for cytological grading of mastocytosis. Leuk Res. 2001; 25: 529-536.
- 9Escribano L, Dı́az-Agustı́n B, Bellas C, et al. Utility of flow cytometric analysis of mast cells in the diagnosis and classification of adult mastocytosis. Leuk Res. 2001; 25: 563-570.
- 10Longley BJ, Reguera MJ, Ma Y. Classes of c-KIT activating mutations: proposed mechanisms of action and implications for disease classification and therapy. Leuk Res. 2001; 25: 571-576.
- 11Schwartz LB, Metcalfe DD, Miller JS, Earl H, Sullivan T. Tryptase levels as an indicator of mast-cell activation in systemic anaphylaxis and mastocytosis. N Engl J Med. 1987; 316: 1622-1626.
- 12Sperr WR, Jordan J-H, Fiegl M, et al. Serum tryptase levels in patients with mastocytosis: correlation with mast cell burden and implication for defining the category of disease. Int Arch Allergy Immunol. 2002; 128: 136-141.
- 13Akin C, Metcalfe DD. Systemic mastocytosis. Annu Rev Med. 2004; 55: 419-432.
- 14Metcalfe DD. Mast cells and mastocytosis. Blood. 2008; 112(4): 946-956.
- 15Valent P, Sperr WR, Akin C. How I treat patients with advanced systemic mastocytosis. Blood. 2010; 116(26): 5812-5817.
- 16Escribano L, Álvarez-Twose I, Sánchez-Muñoz L, et al. Prognosis in adult indolent systemic mastocytosis: a long-term study of the Spanish Network on Mastocytosis in a series of 145 patients. J Allergy Clin Immunol. 2009; 124(3): 514-521.
- 17Sperr WR, Horny HP, Valent P. Spectrum of associated clonal hematologic non-mast cell lineage disorders occurring in patients with systemic mastocytosis. Int Arch Allergy Immunol. 2002; 127(2): 140-142.
- 18Metcalfe DD. Classification and diagnosis of mastocytosis: current status. J Invest Dermatol. 1991; 96(3): 2S-4S.
- 19Valent P, Oude Elberink JNG, Gorska A, et al. The data registry of the European Competence Network on Mastocytosis (ECNM): set up, projects and perspectives. J Allergy Clin Immunol Pract. 2019; 7(1): 81-87.
- 20Lennert K, Parwaresch MR. Mast cells and mast cell neoplasia: a review. Histopathology. 1979; 3(5): 349-365.
- 21Sotlar K, Cerny-Reiterer S, Petat-Dutter K, et al. Aberrant expression of CD30 in neoplastic mast cells in high-grade mastocytosis. Mod Pathol. 2011; 24(4): 585-595.
- 22Valent P, Cerny-Reiterer S, Herrmann H, et al. Phenotypic heterogeneity and target expression profiles of normal and neoplastic mast cells. Best Pract Res Clin Haematol. 2010; 23(3): 369-378.
- 23Escribano L, Orfao A, Dı́az-Agustin B, et al. Indolent systemic mast cell disease in adults: immunophenotypic characterization of bone marrow mast cells and its diagnostic implications. Blood. 1998; 91(8): 2731-2736.
- 24Escribano L, Orfao A, Villarrubia J, et al. Sequential immunophenotypic analysis of mast cells in a case of systemic mast cell disease evolving to a mast cell leukemia. Cytometry. 1997; 30(2): 98-102.
10.1002/(SICI)1097-0320(19970415)30:2<98::AID-CYTO4>3.0.CO;2-9 CAS PubMed Web of Science® Google Scholar
- 25Teodosio C, Mayado A, Sa´nchez-Mun~oz L, et al. The immunophenotype of mast cells and its utility in the diagnostic work-up of systemic mastocytosis. J Leukoc Biol. 2015; 97: 49-59.
- 26Pardanani A, Reichard KK, Zblewski D, et al. CD123 immunostaining patterns in systemic mastocytosis: differential expression in disease subgroups and potential prognostic value. Leukemia. 2016; 30: 914-918.
- 27Pardanani A, Finke C, Abdelrahman RA, et al. Increased circulating IL-2Ra (CD25) predicts poor outcome in both indolent and aggressive forms of mastocytosis: a comprehensive cytokine–phenotype study. Leukemia. 2013; 27: 1430-1433.
- 28Hoermann G, Gleixner KV, Dinu GE, et al. The KIT D816V allele burden predicts survival in patients with mastocytosis and correlates with the WHO type of the disease. Allergy. 2014; 69(6): 810-813.
- 29Schwaab J, Schnittger S, Sotlar K, et al. Comprehensive mutational profiling in advanced systemic mastocytosis. Blood. 2013; 122(14): 2460-2466.
- 30Jawhar M, Schwaab J, Schnittger S, et al. Additional mutations in SRSF2, ASXL1 and/or RUNX1 identify a high-risk group of patients with KIT D816V+ advanced systemic mastocytosis. Leukemia. 2016; 30: 136-143.
- 31Greiner G, Witzeneder N, Berger A, et al. CCL2 is a KIT D816V-dependent modulator of the bone marrow microenvironment in systemic mastocytosis. Blood. 2017; 129(3): 371-382.
- 32Valent P, Arock M, Bonadonna P, et al. European Competence Network on Mastocytosis (ECNM): 10-year jubilee, update, and future perspectives. Wien Klin Wochenschr. 2012; 124(23–24): 807-814.
- 33Hartmann K, Escribano L, Grattan C, et al. Cutaneous manifestations in patients with mastocytosis: Consensus report of the European Competence Network on Mastocytosis; the American Academy of Allergy, Asthma & Immunology; and the European Academy of Allergology and Clinical Immunology. J Allergy Clin Immunol. 2016; 137(1): 35-45.
- 34Pardanani A. Systemic mastocytosis in adults: 2015 update on diagnosis, risk stratification, and management. Am J Hematol. 2015; 90: 250-262.