Immunotherapy with grass pollen tablets reduces medication dispensing for allergic rhinitis and asthma: A retrospective database study in France
Abstract
Background
Moderate-to-severe allergic rhinitis (AR) may increase the risk of developing or worsening asthma, whereas treatment of AR with subcutaneously or sublingual allergen immunotherapy (SLIT) may slow this progression.
Methods
In a retrospective real-world analysis, prescription fulfilment data were gathered from French retail pharmacies between 1 March 2012 and 31 December 2016. Using linear regression analyses, patients having received at least two prescriptions of grass pollen SLIT tablets over at least 2 successive years were compared with control patients having received symptomatic medications only.
Results
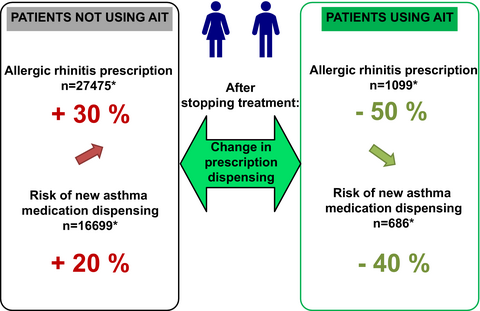
A total of 1099 SLIT patients and 27 475 control patients were included in the main analysis. With regard to symptomatic AR medication dispensing, we observed a 50% decrease in the pre-index/follow-up ratio in the SLIT group, a 30% increase in the control group without age matching (P < 0.0001 vs SLIT) and a 20% increase in the control group with age matching (P < 0.0001 vs SLIT). During the follow-up, 11 (1.8%) and 782 (5.3%) patients initiated asthma treatment in the SLIT and control groups, respectively. The relative risk of medication dispensing for new asthma was lower in the SLIT group (by 62.5% [29.1%-80.1%] without age matching (P = 0.0025) and by 63.7% [31.5%-80.7%] with age matching; P = 0.0018). SLIT was also associated with slower progression of asthma medication dispensing during the follow-up period, relative to the control group (regression coefficient: −0.58 [−0.74 to 0.42] without age matching (P < 0.0001) and −0.61 [−0.76 to −0.46] with age matching; P < 0.0001).
Conclusion
Prescription of grass pollen SLIT tablets reduced the dispensing of AR and asthma medications in real life.
Graphical Abstract
- Patients having received grass pollen sublingual immunotherapy (SLIT) tablets over at least 2 successive years were compared with control patients having received symptomatic medications only. After treatment, the dispensing of symptomatic allergic rhinitis medication (a proxy for disease burden) fell by 50% in the SLIT group and increased by 30% in the control group. When judged with regard to medication dispensing, the risk of new asthma and the progression of existing asthma were lower in the SLIT group than in the control group.
CONFLICTS OF INTEREST
X. Ansolabehere, I. Bardoulat, N. Coulombel, F. Maurel and P. Le Jeunne are salaried employees of IQVIA (La Défense, France). P Devillier has received fees for lectures and advisory boards from ALK, Astra Zeneca, Boehringer Ingelheim, Chiesi, GlaxoSmithKline, IMS Health GmbH & Co OHG, Meda Pharma, Menarini, Novartis, Nycomed-Takeda, Sandoz, Stallergenes Greer and Teva outside the submitted work. M Molimard is employed by the University of Bordeaux France and reports funding form ALK, Boehringer Ingelheim, IQVIA, GSK, Novartis Pharma and Stallergenes Greer outside the submitted work. P. Demoly reports personal fees from ALK, Stallergenes Greer, Chiesi, Thermo Fisher Scientific, Ménarini, Bausch&Lomb and Mylan, outside the submitted work.