Chitotriosidase inhibits allergic asthmatic airways via regulation of TGF-β expression and Foxp3+ Treg cells
J. Y. Hong
Department of Pediatrics and Institute of Allergy, Brain Korea 21 PLUS Project for Medical Science, Yonsei University College of Medicine, Seoul, Korea
Search for more papers by this authorM. Kim
Department of Pediatrics and Institute of Allergy, Brain Korea 21 PLUS Project for Medical Science, Yonsei University College of Medicine, Seoul, Korea
Search for more papers by this authorI. S. Sol
Department of Pediatrics and Institute of Allergy, Brain Korea 21 PLUS Project for Medical Science, Yonsei University College of Medicine, Seoul, Korea
Search for more papers by this authorK. W. Kim
Department of Pediatrics and Institute of Allergy, Brain Korea 21 PLUS Project for Medical Science, Yonsei University College of Medicine, Seoul, Korea
Search for more papers by this authorC.-M. Lee
Molecular Microbiology and Immunology, Brown University, Providence, RI, USA
Search for more papers by this authorJ. A. Elias
Molecular Microbiology and Immunology, Brown University, Providence, RI, USA
Search for more papers by this authorCorresponding Author
M. H. Sohn
Department of Pediatrics and Institute of Allergy, Brain Korea 21 PLUS Project for Medical Science, Yonsei University College of Medicine, Seoul, Korea
Correspondence
Chun Geun Lee, Molecular Microbiology and Immunology, Brown University, Providence, RI, USA.
Email: [email protected]
and
Myung Hyun Sohn, Department of Pediatrics and Institute of Allergy, Yonsei University College of Medicine, Seodaemun-gu, Seoul, Korea.
Email: [email protected]
Search for more papers by this authorCorresponding Author
C. G. Lee
Molecular Microbiology and Immunology, Brown University, Providence, RI, USA
Department of Internal Medicine, Hanyang University, Seoul, Korea
Correspondence
Chun Geun Lee, Molecular Microbiology and Immunology, Brown University, Providence, RI, USA.
Email: [email protected]
and
Myung Hyun Sohn, Department of Pediatrics and Institute of Allergy, Yonsei University College of Medicine, Seodaemun-gu, Seoul, Korea.
Email: [email protected]
Search for more papers by this authorJ. Y. Hong
Department of Pediatrics and Institute of Allergy, Brain Korea 21 PLUS Project for Medical Science, Yonsei University College of Medicine, Seoul, Korea
Search for more papers by this authorM. Kim
Department of Pediatrics and Institute of Allergy, Brain Korea 21 PLUS Project for Medical Science, Yonsei University College of Medicine, Seoul, Korea
Search for more papers by this authorI. S. Sol
Department of Pediatrics and Institute of Allergy, Brain Korea 21 PLUS Project for Medical Science, Yonsei University College of Medicine, Seoul, Korea
Search for more papers by this authorK. W. Kim
Department of Pediatrics and Institute of Allergy, Brain Korea 21 PLUS Project for Medical Science, Yonsei University College of Medicine, Seoul, Korea
Search for more papers by this authorC.-M. Lee
Molecular Microbiology and Immunology, Brown University, Providence, RI, USA
Search for more papers by this authorJ. A. Elias
Molecular Microbiology and Immunology, Brown University, Providence, RI, USA
Search for more papers by this authorCorresponding Author
M. H. Sohn
Department of Pediatrics and Institute of Allergy, Brain Korea 21 PLUS Project for Medical Science, Yonsei University College of Medicine, Seoul, Korea
Correspondence
Chun Geun Lee, Molecular Microbiology and Immunology, Brown University, Providence, RI, USA.
Email: [email protected]
and
Myung Hyun Sohn, Department of Pediatrics and Institute of Allergy, Yonsei University College of Medicine, Seodaemun-gu, Seoul, Korea.
Email: [email protected]
Search for more papers by this authorCorresponding Author
C. G. Lee
Molecular Microbiology and Immunology, Brown University, Providence, RI, USA
Department of Internal Medicine, Hanyang University, Seoul, Korea
Correspondence
Chun Geun Lee, Molecular Microbiology and Immunology, Brown University, Providence, RI, USA.
Email: [email protected]
and
Myung Hyun Sohn, Department of Pediatrics and Institute of Allergy, Yonsei University College of Medicine, Seodaemun-gu, Seoul, Korea.
Email: [email protected]
Search for more papers by this authorAbstract
Background
Chitotriosidase (chitinase 1, Chit1), a major true chitinase in humans, is induced in childhood asthma and has been implicated in the pathogenesis of a variety of inflammatory and tissue remodeling responses. However, the role and the mechanisms that underlie these contributions to the diseases have not been defined. We hypothesized that Chit1 plays a significant role in the pathogenesis of allergic asthma.
Methods
Wild-type and Chit1-deficient mice and cells in culture were used to define the roles of Chit1 in models of allergic adaptive Th2 inflammation. In addition, the levels of sputum Chit1 were evaluated in pediatric asthma patients and compared to control.
Results
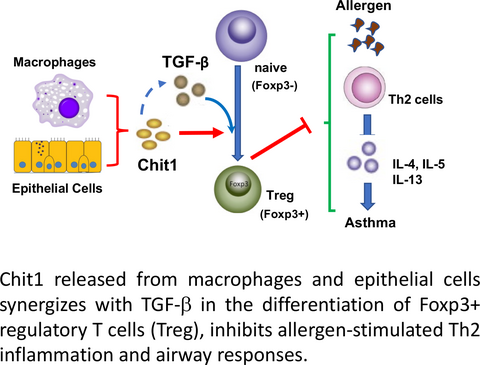
The levels of sputum Chit1 were significantly increased in the patients with childhood asthma. Mice with Chit1 null mutation demonstrated enhanced allergic Th2 inflammatory and cytokine and IgE responses to OVA or house dust mite allergen sensitization and challenge. However, the expression levels of TGF-β1 were significantly decreased with a diminished number of Foxp3+ regulatory T cells (Treg) in the lungs of Chit1−/− mice compared to WT controls. In vitro, the absence of Chit1 significantly reduced TGF-β-stimulated conversion of CD4+CD25− naïve T cells to CD4+Foxp3+ Treg cells, suggesting Chit1 is required for optimal effect of TGF-β1 in Treg cell differentiation.
Conclusion
Chit1 plays a protective role in the pathogenesis of allergic inflammation and asthmatic airway responses via regulation of TGF-β expression and Foxp3+ Treg cells.
Graphical Abstract
CONFLICTS OF INTEREST
The authors declare that they have no conflicts of interest.
Supporting Information
| Filename | Description |
|---|---|
| all13426-sup-0001-Supinfo.docxWord document, 571.1 KB |
Please note: The publisher is not responsible for the content or functionality of any supporting information supplied by the authors. Any queries (other than missing content) should be directed to the corresponding author for the article.
REFERENCES
- 1Akinbami LJ, Moorman JE, Bailey C, et al. Trends in asthma prevalence, health care use, and mortality in the United States, 2001-2010. NCHS Data Brief. 2012; 94: 1-8.
- 2Cruz AA, Bousquet J, Khaltaev N. Global Surveillance, Prevention and Control of Chronic Respiratory Diseases: A Comprehensive Approach. Switzerland: World Health Organization; 2007.
- 3Fahy JV. Type 2 inflammation in asthma–present in most, absent in many. Nat Rev Immunol. 2015; 15: 57-65.
- 4Lee CG, Da Silva CA, Dela Cruz CS, et al. Role of chitin and chitinase/chitinase-like proteins in inflammation, tissue remodeling, and injury. Annu Rev Physiol. 2011; 73: 479-501.
- 5Wiesner DL, Specht CA, Lee CK, et al. Chitin recognition via chitotriosidase promotes pathologic type-2 helper T cell responses to cryptococcal infection. PLoS Pathog. 2015; 11: e1004701.
- 6Zhu Z, Zheng T, Homer RJ, et al. Acidic mammalian chitinase in asthmatic Th2 inflammation and IL-13 pathway activation. Science. 2004; 304: 1678-1682.
- 7Vicencio AG, Chupp GL, Tsirilakis K, et al. CHIT1 mutations: genetic risk factor for severe asthma with fungal sensitization? Pediatrics. 2010; 126: e982-e985.
- 8Lee CG, Herzog EL, Ahangari F, et al. Chitinase 1 is a biomarker for and therapeutic target in scleroderma-associated interstitial lung disease that augments TGF-β1 signaling. J Immunol. 2012; 189: 2635-2644.
- 9Kim KW, Park J, Lee JH, et al. Association of genetic variation in chitotriosidase with atopy in Korean children. Ann Allergy Asthma Immunol. 2013; 110: 444-449.
- 10Seibold MA, Donnelly S, Solon M, et al. Chitotriosidase is the primary active chitinase in the human lung and is modulated by genotype and smoking habit. J Allergy Clin Immunol. 2008; 122: 944-950.
- 11Sakaguchi S, Yamaguchi T, Nomura T, Ono M. Regulatory T cells and immune tolerance. Cell. 2008; 133: 775-787.
- 12Selvaraj RK, Geiger TL. A kinetic and dynamic analysis of Foxp3 induced in T cells by TGF-beta. J Immunol. 2007; 178: 7667-7677.
- 13Lee CG, Cho SJ, Kang MJ, et al. Early growth response gene 1-mediated apoptosis is essential for transforming growth factor beta1-induced pulmonary fibrosis. J Exp Med. 2004; 200: 377-389.
- 14Knosp CA, Schiering C, Spence S, et al. Regulation of Foxp3 + inducible regulatory T cell stability by SOCS2. J Immunol. 2013; 190: 3235-3245.
- 15Fantini MC, Becker C, Monteleone G, Pallone F, Galle PR, Neurath MF. Cutting edge: TGF-beta induces a regulatory phenotype in CD4 + CD25- T cells through Foxp3 induction and down-regulation of Smad7. J Immunol. 2004; 172: 5149-5153.
- 16 National Asthma Education and Prevention Program. Expert Panel Report 3 (EPR-3): Guidelines for the Diagnosis and Management of Asthma-Summary Report 2007. J Allergy Clin Immunol. 2007; 120(5 Suppl): S94-S138.
- 17Kim LK, Morita R, Kobayashi Y, et al. AMCase is a crucial regulator of type 2 immune responses to inhaled house dust mites. Proc Natl Acad Sci U S A. 2015; 112: E2891-E2899.
- 18Lee CG, Hartl D, Lee GR, et al. Role of breast regression protein 39 (BRP-39)/chitinase 3-like-1 in Th2 and IL-13-induced tissue responses and apoptosis. J Exp Med. 2009; 206: 1149-1166.
- 19Cho SJ, Weiden MD, Lee CG. Chitotriosidase in the pathogenesis of inflammation, interstitial lung diseases and COPD. Allergy Asthma Immunol Res. 2015; 7: 14-21.
- 20van Eijk M, van Roomen CP, Renkema GH, et al. Characterization of human phagocyte-derived chitotriosidase, a component of innate immunity. Int Immunol. 2005; 17: 1505-1512.
- 21Elmonem MA, van den Heuvel LP, Levtchenko EN. Immunomodulatory effects of chitotriosidase enzyme. Enzyme Res. 2016; 2016: 2682680.
- 22Kanneganti M, Kamba A, Mizoguchi E. Role of chitotriosidase (chitinase 1) under normal and disease conditions. J Epithel Biol Pharmacol. 2012; 5: 1-9.
- 23James AJ, Reinius LE, Verhoek M, et al. Increased YKL-40 and chitotriosidase in asthma and chronic obstructive pulmonary disease. Am J Respir Crit Care Med. 2016; 193: 131-142.
- 24Bargagli E, Olivieri C, Margollicci M, et al. Serum chitotriosidase levels in patients with allergic and non-allergic asthma. Respiration. 2010; 79: 437-438.
- 25Pesce J, Kaviratne M, Ramalingam TR, et al. The IL-21 receptor augments Th2 effector function and alternative macrophage activation. J Clin Invest. 2006; 116: 2044-2055.
- 26Migliaccio CT, Buford MC, Jessop F, Holian A. The IL-4Ralpha pathway in macrophages and its potential role in silica-induced pulmonary fibrosis. J Leukoc Biol. 2008; 83: 630-639.
- 27Carambia A, Freund B, Schwinge D, et al. TGF-β-dependent induction of CD4⁺CD25⁺Foxp3⁺ Tregs by liver sinusoidal endothelial cells. J Hepatol. 2014; 61: 594-599.
- 28Chen W, Jin W, Hardegen N, et al. Conversion of peripheral CD4 + CD25- naive T cells to CD4 + CD25 + regulatory T cells by TGF-beta induction of transcription factor Foxp3. J Exp Med. 2003; 198: 1875-1886.
- 29Bettelli E, Carrier Y, Gao W, et al. Reciprocal developmental pathways for the generation of pathogenic effector TH17 and regulatory T cells. Nature. 2006; 441: 235-238.
- 30Tran DQ, Ramsey H, Shevach EM. Induction of FOXP3 expression in naive human CD4 + FOXP3 T cells by T-cell receptor stimulation is transforming growth factor-beta dependent but does not confer a regulatory phenotype. Blood. 2007; 110: 2983-2990.
- 31Wan Y, Flavell RA. Regulatory T cells, transforming growth factor-beta, and immune suppression. Proc Am Thorac Soc. 2007; 4: 271-276.
- 32Brown SD, Baxter KM, Stephenson ST, Esper AM, Brown LA, Fitzpatrick AM. Airway TGF-β1 and oxidant stress in children with severe asthma: association with airflow limitation. J Allergy Clin Immunol. 2012; 129: 388-396.
- 33Minshall EM, Leung DY, Martin RJ, et al. Eosinophil-associated TGF-beta1 mRNA expression and airways fibrosis in bronchial asthma. Am J Respir Cell Mol Biol. 1997; 17: 326-333.
- 34Chakir J, Shannon J, Molet S, et al. Airway remodeling-associated mediators in moderate to severe asthma: effect of steroids on TGF-beta, IL-11, IL-17, and type I and type III collagen expression. J Allergy Clin Immunol. 2003; 111: 1293-1298.
- 35Cobbold SP, Castejon R, Adams E, et al. Induction of foxP3 + regulatory T cells in the periphery of T cell receptor transgenic mice tolerized to transplants. J Immunol. 2004; 172: 6003-6010.
- 36Luo X, Yang H, Kim IS, et al. Systemic transforming growth factor-beta1 gene therapy induces Foxp3 + regulatory cells, restores self-tolerance, and facilitates regeneration of beta cell function in overtly diabetic nonobese diabetic mice. Transplantation. 2005; 79: 1091-1096.
- 37Dardalhon V, Awasthi A, Kwon H, et al. IL-4 inhibits TGF-beta-induced Foxp3 + T cells and together with TGF-beta, generates IL-9 + IL-10 + Foxp3(-) effector T cells. Nat Immunol. 2008; 9: 1347-1355.
- 38Kastner L, Dwyer D, Qin FX. Synergistic effect of IL-6 and IL-4 in driving fate revision of natural Foxp3 + regulatory T cells. J Immunol. 2010; 185: 5778-5786.
- 39Coleman M, Ruane D, Moran B, Dunne PJ, Keane J, Mills KH. Alveolar macrophages contribute to respiratory tolerance by inducing FoxP3 expression in naive T cells. Am J Respir Cell Mol Biol. 2013; 48: 773-780.
- 40Soroosh P, Doherty TA, Duan W, et al. Lung-resident tissue macrophages generate Foxp3 + regulatory T cells and promote airway tolerance. J Exp Med. 2013; 210: 775-788.
- 41Da Silva CA, Chalouni C, Williams A, Hartl D, Lee CG, Elias JA. Chitin is a size-dependent regulator of macrophage TNF and IL-10 production. J Immunol. 2009; 182: 3573-3582.
- 42Choi JP, Lee SM, Choi HI, et al. House dust mite-derived chitin enhances Th2 cell response to inhaled allergens, mainly via a TNF-alpha-dependent pathway. Allergy Asthma Immunol Res. 2016; 8: 362-374.
- 43Dubey LK, Moeller JB, Schlosser A, Sorensen GL, Holmskov U. Chitin enhances serum IgE in Aspergillus fumigatus induced allergy in mice. Immunobiology. 2015; 220: 714-721.
- 44Lee CG, Da Silva CA, Lee J, Hartl D, Elias JA. Chitin regulation of immune responses: an old molecule with new roles. Curr Opin Immunol. 2008; 20: 684-689.
- 45Wagener J, Malireddi RK, Lenardon MD, et al. Fungal chitin dampens inflammation through IL-10 induction mediated by NOD2 and TLR9 activation. PLoS Pathog. 2014; 10: e1004050.
- 46Reese TA, Liang HE, Tager AM, et al. Chitin induces accumulation in tissue of innate immune cells associated with allergy. Nature. 2007; 447: 92-96.
- 47Shibata Y, Foster LA, Bradfield JF, Myrvik QN. Oral administration of chitin down-regulates serum IgE levels and lung eosinophilia in the allergic mouse. J Immunol. 2000; 164: 1314-1321.