Theoretical study of cooperative effects in the homo- and heteromeric hydrogen bond chains (HCN)nHF with n = 1, 2, and 3
Abstract
Theoretical investigations concerning the formation of hydrogen bonds in both homomeric, (HCN)n, and heteromeric clusters of the type (HCN)nHF (n = 1, 2, and 3) have been performed through ab initio molecular orbital calculations at the second-order Møller–Plesset (MP2) and density functional theory (DFT)/B3LYP levels, with the 6-311++G(d,p) basis set. The formation of hydrogen bonds is investigated in terms of changes in structural, electronic, and vibrational parameters of the free species. Important parameters include the increment in the distance of the HF proton donor species, the increment in the HC distance of HCN moiety, and the amount of intermolecular charge transfer between the HCN species in the (HCN)n group. It is interesting to point out the different behavior in the HC distance as HCN acts simultaneously as a proton acceptor, proton donor, and a proton donor and acceptor. Other important results concern the cooperative effect (CE) in terms of the stabilization energy and dipole moment. Both CEs increase with cluster size and are more pronounced for the heteroclusters. The HF stretching frequency is red-shifted on going in the direction (HCN)HF → (HCN)2HF → (HCN)3HF. This trend is in agreement with the following order of stabilization energies: ΔEHCN…HF < ΔE < ΔE
< ΔE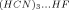 . © 2006 Wiley Periodicals, Inc. Int J Quantum Chem, 2006
. © 2006 Wiley Periodicals, Inc. Int J Quantum Chem, 2006




