Molecular dynamics simulation study of thermomechanical properties and hydrogen bonding structures of two-component polyurethanes
Abstract
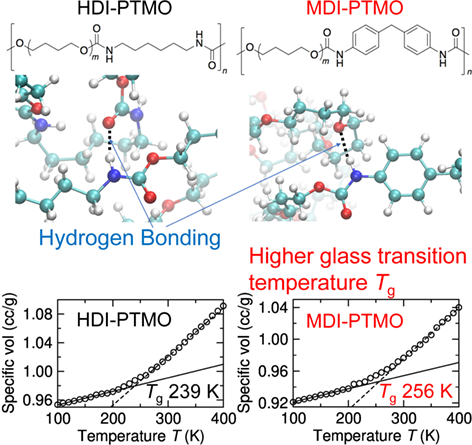
We investigated the thermomechanical properties and hydrogen bonding (HB) structures of two-component hexamethylenediisocyanate (HDI)-poly(tetramethylene oxide) (PTMO) and 4,4′-methylenediphenyldiisocyanate (MDI)-PTMO polyurethanes with atomistic molecular dynamics simulations. We used different temperature-scanning and sampling protocols to estimate the glass transition temperature (Tg) of these two polyurethanes. Our simulation results consistently predicted higher Tg values for MDI-PTMO than those for HDI-PTMO, in qualitative agreement with the same trend observed in experiments. We also studied two different types of HB interactions in these polyurethanes. At ambient temperature, more HB contacts between amide-hydrogens and carbonyl-oxygens of the carbamate groups along with longer lifetimes were noted in HDI-PTMO than in MDI-PTMO, while the latter revealed more HB contacts between amide-hydrogens and ether-oxygens of PTMO. MDI-PTMO, encompassing more rigid chain structures, displayed higher stress values in stress–strain profiles from high strain-rate tensile deformations at ambient temperature than HDI-PTMO, which is consistent with a higher Tg value of MDI-PTMO.