Photobiomodulation therapy promotes in vitro wound healing in nicastrin KO HaCaT cells
Corresponding Author
Paola M. Tricarico
University of Trieste, Trieste, Italy
Correspondence
Paola M. Tricarico, University of Trieste, Piazzale Europa 1, 34138 Trieste, Italy.
Email: [email protected]
Search for more papers by this authorFrancette Jean-Louis
INSERM U955 Eq.16, Institut Mondor de Recherche Biomédicale and VRI (Vaccine Research Institute), Créteil, France
Search for more papers by this authorMichele Boniotto
INSERM U955 Eq. 16, Institut Mondor de Recherche Biomédicale and Université Paris Est-Créteil (UPEC), Faculté de Médecine, Créteil, France
Search for more papers by this authorSergio Crovella
University of Trieste, Trieste, Italy
Institute for Maternal and Child Health "Burlo Garofolo", Trieste, Italy
Search for more papers by this authorCorresponding Author
Paola M. Tricarico
University of Trieste, Trieste, Italy
Correspondence
Paola M. Tricarico, University of Trieste, Piazzale Europa 1, 34138 Trieste, Italy.
Email: [email protected]
Search for more papers by this authorFrancette Jean-Louis
INSERM U955 Eq.16, Institut Mondor de Recherche Biomédicale and VRI (Vaccine Research Institute), Créteil, France
Search for more papers by this authorMichele Boniotto
INSERM U955 Eq. 16, Institut Mondor de Recherche Biomédicale and Université Paris Est-Créteil (UPEC), Faculté de Médecine, Créteil, France
Search for more papers by this authorSergio Crovella
University of Trieste, Trieste, Italy
Institute for Maternal and Child Health "Burlo Garofolo", Trieste, Italy
Search for more papers by this authorAbstract
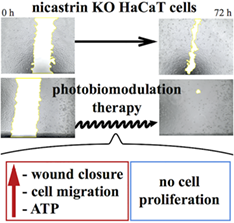
Mutations in NCSTN gene (encoding for nicastrin protein) are associated with hidradenitis suppurativa (HS), a chronic inflammatory disease involving hair follicles. HS is clinically handled with drugs but the most severe cases are treated with surgery. Photobiomodulation (PBM) therapy, already used in the treatment of skin diseases such as acne, herpes virus lesions, ultraviolet damage, vitiligo, hypertrophic scar, keloid, burn, psoriasis and diabetic chronic wounds, could be beneficial as an adjuvant supportive treatment to promote and foster the healing process after skin excision in HS. The effects of PBM therapy in promoting the wound closure are evaluated in a HaCaT cells NCSTN−/−, assessing cell metabolism, migration rate, proliferation and cell cycle progression. In our experimental model, PBM exerts a potent action on metabolism of mutated keratinocytes, incrementing adenosine triphosphate (ATP) production at 2 hours, while after 24 hours an increase of metabolism with a decrement of intracellular ATP levels were recorded. Moreover, PBM speeds up the wound closure, inducing cells' migration without affecting their proliferation.Based on our findings, we suggest the use of PBM in HS patients, who undergo major surgery with large skin excision.
Supporting Information
| Filename | Description |
|---|---|
| jbio201800174-sup-0001-author-biographies.docxapplication/docx, 377.5 KB | Author Biographies |
| jbio201800174-sup-0002-AppendixS1.docxWord 2007 document , 377.5 KB |
Appendix S1 Supporting Information |
| jbio201800174-sup-0003-FigureS1.docxWord 2007 document , 324.4 KB |
Figure S1 HaCaT cells NCSTN−/−. Representative Western blots images showing levels of beta-actin and nicastrin, the first used as the reference protein, in HaCaT cells NCSTN−/− and HaCaT cells wild type (WT). |
Please note: The publisher is not responsible for the content or functionality of any supporting information supplied by the authors. Any queries (other than missing content) should be directed to the corresponding author for the article.
REFERENCES
- 1G. B. Jemec, J. Cutan. Med. Surg. 2003, 7, 47.
- 2W. Chen, G. Plewig, Exp. Dermatol. 2017, 26, 544.
- 3C. Liy-Wong, E. Pope, I. Lara-Corrales, J. Am. Acad. Dermatol. 2015, 73, S36.
- 4J. M. von der Werth, H. C. Williams, J. Eur. Acad. Dermatol. Venereol. 2000, 14, 389.
- 5M. J. Anderson, M. B. Dockerty, Dis. Colon Rectum 1958, 1, 23.
- 6J. Gasparic, P. Theut Riis, G. B. Jemec, J. Eur. Acad. Dermatol. Venereol. 2017, 31, 1809.
- 7B. Wang, W. Yang, W. Wen, J. Sun, B. Su, B. Liu, et al., Science 2010, 19, 330.
- 8X. Xiao, Y. He, C. Li, X. Zhang, H. Xu, B. Wang, Br. J. Dermatol. 2016, 174, 522.
- 9J. R. Ingram, Dermatol. Clin. 2016, 34, 23.
- 10A. V. Marzano, I. Ceccherini, M. Gattorno, D. Fanoni, F. Caroli, M. Rusmini, A. Grossi, C. De Simone, O. M. Borghi, P. L. Meroni, C. Crosti, M. Cugno, Medicine 2014, 93, e187.
- 11A. V. Marzano, V. Trevisan, M. Gattorno, I. Ceccherini, C. De Simone, C. Crosti, JAMA Dermatol. 2013, 149, 762.
- 12A. V. Marzano, G. Damiani, I. Ceccherini, E. Berti, M. Gattorno, M. Cugno, Br. J. Dermatol. 2017, 176, 1588.
- 13Y. Pan, M. H. Lin, X. Tian, H. T. Cheng, T. Gridley, J. Shen, R. Kopan, Dev. Cell 2004, 7, 731.
- 14G. Gener, F. Canoui-Poitrine, J. E. Revuz, O. Faye, F. Poli, G. Gabison, et al., Dermatology 2009, 219, 148.
- 15H. H. van der Zee, J. Boer, E. P. Prens, G. B. Jemec, Dermatology 2009, 219, 143.
- 16Y. E. Chen, T. Gerstle, K. Verma, M. D. Treiser, A. B. Kimball, D. P. Orgill, Plast. Reconstr. Surg. 2014, 133, 370.
- 17C. C. Johnstone, A. Farley, C. Hendry, Nurs. Standard. 2005, 19, 59.
- 18A. B. Kimball, M. H. Gold, B. Zib, M. W. Davis, J. Am. Acad. Dermatol. 2008, 59, 448.
- 19V. Dini, T. Oranges, L. Rotella, M. Romanelli, J. Low Extrem. Wounds 2015, 14, 236.
- 20R. Parrado, M. Cadena, A. Vergara, D. Cadena, J. G. Chalela, Int. Wound J. 2017, 14, 35.
- 21P. Avci, A. Gupta, M. Sadasivam, D. Vecchio, Z. Pam, N. Pam, M. R. Hamblin, Semin. Cutan. Med. Surg. 2013, 32, 41.
- 22H. Chung, T. Dai, S. K. Sharma, Y. Y. Huang, J. D. Carroll, M. R. Hamblin, Ann. Biomed. Eng. 2012, 40, 516.
- 23A. Gupta, P. Avci, M. Sadasivam, R. Chandran, N. Parizotto, D. Vecchio, W. C. de Melo, T. Dai, L. Y. Chiang, M. R. Hamblin, Biotechnol. Adv. 2013, 31, 607.
- 24T. I. Karu, S. F. Kolyakov, Photomed. Laser Surg. 2005, 23, 355.
- 25M. Greco, G. Guida, E. Perlino, E. Marra, E. Quagliariello, Biochem. Biophys. Res. Commun. 1988, 163, 1428.
- 26L. F. de Freitas, H. M. Ramblin, IEEE J. Sel. Top. Quantum Electron. 2016, 22.
- 27A. E. Saltmarche, Int. Wound J. 2008, 5, 351.
- 28N. E. Sanjana, O. Shalem, F. Zhang, Nat. Methods 2014, 11, 783.
- 29L. T. Fox, A. Mazumder, A. Dwivedi, M. Gerber, J. du Plessis, J. H. Hamman, J. Ethnopharmacol. 2017, 22, 200.
- 30G. Ottaviani, M. Gobbo, M. Sturnega, V. Martinelli, M. Mano, F. Zanconati, R. Bussani, G. Perinetti, C. S. Long, R. Di Lenarda, M. Giacca, M. Biasotto, S. Zacchigna, Am. J. Pathol. 2013, 183, 1747.
- 31S. Farivar, T. Malekshahabi, R. Shiari, J. Lasers Med. Sci. 2014, 5, 58.
- 32G. Ottaviani, V. Martinelli, K. Rupel, N. Caronni, A. Naseem, L. Zandonà, G. Perinetti, M. Gobbo, R. Di Lenarda, R. Bussani, F. Benvenuti, M. Giacca, M. Biasotto, S. Zacchigna, EBioMedicine 2016, 11, 165.
- 33D. H. H. Abrahamse, Photomed. Laser Surg. 2005, 23, 251.
- 34T. Karu, J. Photochem. Photobiol. B 1987, 3, 638.
10.1016/1011-1344(89)80088-0 Google Scholar
- 35T. Karu, IUBMB Life 2010, 62, 607.
- 36T. Karu, L. Pyatibrat, G. Kalendo, J. Photochem. Photobiol. B 1995, 27, 219.
- 37G. Y. Lomakina, Y. A. Modestova, N. N. Ugarova, Biochemistry 2015, 80, 701.
- 38M. Wickersham, S. Wachtel, T. Wong Fok Lung, G. Soong, R. Jacquet, A. Richardson, D. Parker, A. Prince, Cell Rep. 2017, 18, 2742.
- 39H. Guet-Revillet, J. P. Jais, M. N. Ungeheuer, H. Coignard-Biehler, S. Duchatelet, M. Delage, T. Lam, A. Hovnanian, O. Lortholary, X. Nassif, A. Nassif, O. Join-Lambert, Clin. Infect. Dis. 2017, 65, 282.
- 40G. Kelly, E. P. Prens, Dermatol. Clin. 2016, 34, 51.
- 41I. Pastar, O. Stojadinovic, N. C. Yin, H. Ramirez, A. G. Nusbaum, A. Sawaya, S. B. Patel, L. Khalid, R. R. Isseroff, M. Tomic-Canic, Adv. Wound Care 2014, 3, 445.
10.1089/wound.2013.0473 Google Scholar
- 42R. K. Sivamani, Adv. Wound Care 2014, 3, 476.
10.1089/wound.2014.0523 Google Scholar
- 43D. Jones, A. Banerjee, P. Z. Berger, A. Gross, S. McNish, R. Amdur, V. K. Shanmugam, Immunol. Invest. 2018, 47, 57.
- 44A. P. Sawaya, I. Pastar, O. Stojadinovic, S. Lazovi, S. C. Davis, J. Gil, R. S. Kirsner, M. Tomic-Canic, J. Biol. Chem. 2018, 293, 1439.
- 45G. A. Borges, S. T. Elias, S. M. da Silva, P. O. Magalhães, S. B. Macedo, A. P. Ribeiro, E. N. Guerra, J. Craniomaxillofac. Surg. 2017, 45, 364.
- 46P. Wang, S. M. Henning, D. Heber, PLoS One 2010, 16, 5, e10202.
- 47J. C. Stockert, A. Blázquez-Castro, M. Cañete, R. W. Horobin, A. Villanueva, Acta Histochem. 2012, 114, 796.
- 48E. H. Loreti, V. L. Pascoal, B. V. Nogueira, I. V. Silva, D. F. Pedrosa, Photomed. Laser Surg. 2015, 33, 104.
- 49M. Rizzi, M. Migliario, S. Tonello, V. Rocchetti, F. Renò, Lasers Med. Sci. 2018, 33, 1003.
- 50X. Gao, D. Xing, J. Biomed. Sci. 2009, 16, 4.
- 51F. G. Basso, T. N. Pansani, A. P. S. Turrioni, V. S. Bagnato, J. Hebling, C. A. Souza Costa, Int. J. Dent. 2012, 719452, 1.
10.1155/2012/719452 Google Scholar
- 52F. G. Basso, C. F. Oliveira, C. Kurachi, J. Hebling, C. A. de Souza Costa, Lasers Med. Sci. 2013, 28, 367.
- 53K. M. AlGhamdi, A. Kumar, N. A. Moussa, Lasers Med. Sci. 2012, 27, 237.
- 54A. C. Pellicioli, M. D. Martins, C. S. Dillenburg, M. M. Marques, C. H. Squarize, R. M. Castilho, J. Biomed. Opt. 2014, 19, 028002.
- 55S. W. Jere, N. N. Houreld, H. Abrahamse, J. Photochem. Photobiol. B 2018, 179, 74.
- 56S. W. Jere, H. Abrahamse, N. N. Houreld, Cytokine Growth Factor Rev. 2017, 38, 73.
- 57I. E. Deckers, H. H. van der Zee, J. Boer, E. P. Prens, J. Am. Acad. Dermatol. 2015, 72, 485.
- 58N. Scheinfeld, Clin. Dermatol. 2015, 33, 316.
- 59Y. M. Mengesha, T. C. Holcombe, R. C. Hansen, Pediatr. Dermatol. 1999, 16, 292.




