A targeting nanoplatform for chemo-photothermal synergistic therapy of small-cell lung cancer
Abstract
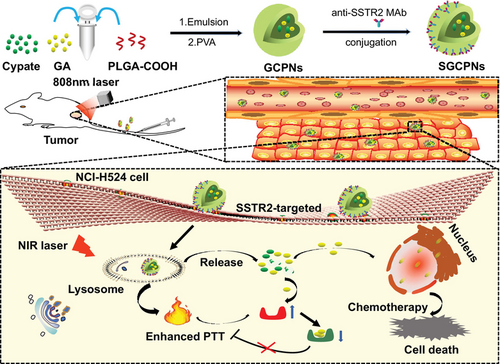
The precise delivery of drugs to tumor sites and the thermoresistance of tumors remain major challenges in photothermal therapy (PTT). Somatostatin receptor 2 (SSTR2) is proposed as an ideal target for the precise treatment of SCLC. We developed a targeting nano-drug delivery system comprising anti-SSTR2 monoclonal antibody (MAb) surface-modified nanoparticles co-encapsulating Cypate and gambogic acid (GA). The formed SGCPNs demonstrated excellent monodispersity, physiological stability, preferable biocompatibility, and resultant efficient photothermal conversion efficacy. SGCPNs were quickly internalized by SSTR2-overexpressing SCLC cells, triggering the release of GA under acidic and near-infrared (NIR) laser irradiation environments, leading to their escape from lysosomes to the cytosol and then diffusion into the nucleus. SGCPNs can not only decrease the cell survival rate but also inhibit the activity of heat shock protein 90 (HSP90). SGCPNs can be precisely delivered to xenograft tumors of SSTR2-positive SCLC in vivo. Upon NIR laser irradiation, therapy of SGCPNs showed significant tumor regression. In conclusion, SGCPNs provide a new chemo-photothermal synergistic treatment strategy for targeting SCLC.
Graphical Abstract
What's new?
Multifunctional nanosystems that combine drug and photothermal therapy (PTT) are promising anticancer treatment strategies. Here, the authors investigated the therapeutic potential of SGCPN, a novel nanoparticle drug-delivery system that combines the PTT dye cypate and the antitumor drug gambogic acid (GA), with somatostatin receptor 2 (SSTR2)-specific targeting capability imparted by antibodies on the nanoparticle surface. SGCPNs were readily internalized by SSTR2-positive small cell lung cancer (SCLC) cells and effectively targeted SSTR2-positive xenograft tumors. In vivo, SGCPNs were precisely delivered to deep-seated SCLC tumor sites and overcame depth-attenuation of light, with near-infrared laser irradiation triggering GA release and tumor cell death.
CONFLICT OF INTEREST STATEMENT
The authors declare that they have no conflict of interest.
Open Research
DATA AVAILABILITY STATEMENT
The data that support the findings of this study are available from the corresponding author upon reasonable request.