Systemic inflammation influences the prognosis of patients with radically resected non-small cell lung cancer and correlates with the immunosuppressive microenvironment
Peiyu Wang
Thoracic Oncology Institute/Department of Thoracic Surgery, Peking University People's Hospital, Beijing, China
Search for more papers by this authorShaodong Wang
Thoracic Oncology Institute/Department of Thoracic Surgery, Peking University People's Hospital, Beijing, China
Search for more papers by this authorZewen Sun
Thoracic Oncology Institute/Department of Thoracic Surgery, Peking University People's Hospital, Beijing, China
Search for more papers by this authorHao Li
Thoracic Oncology Institute/Department of Thoracic Surgery, Peking University People's Hospital, Beijing, China
Search for more papers by this authorYaxing Zhao
Infinity Scope Biotechnology Co. Ltd., Hangzhou, China
Search for more papers by this authorYun Li
Thoracic Oncology Institute/Department of Thoracic Surgery, Peking University People's Hospital, Beijing, China
Search for more papers by this authorFan Yang
Thoracic Oncology Institute/Department of Thoracic Surgery, Peking University People's Hospital, Beijing, China
Search for more papers by this authorJun Wang
Thoracic Oncology Institute/Department of Thoracic Surgery, Peking University People's Hospital, Beijing, China
Search for more papers by this authorKezhong Chen
Thoracic Oncology Institute/Department of Thoracic Surgery, Peking University People's Hospital, Beijing, China
Search for more papers by this authorCorresponding Author
Mantang Qiu
Thoracic Oncology Institute/Department of Thoracic Surgery, Peking University People's Hospital, Beijing, China
Correspondence
Mantang Qiu and Xiao Li, Thoracic Oncology Institute/Department of Thoracic Surgery, Peking University People's Hospital, 11 Xizhimen South Street, Beijing 100044, China.
Email: [email protected], [email protected], and [email protected], [email protected]
Search for more papers by this authorCorresponding Author
Xiao Li
Thoracic Oncology Institute/Department of Thoracic Surgery, Peking University People's Hospital, Beijing, China
Correspondence
Mantang Qiu and Xiao Li, Thoracic Oncology Institute/Department of Thoracic Surgery, Peking University People's Hospital, 11 Xizhimen South Street, Beijing 100044, China.
Email: [email protected], [email protected], and [email protected], [email protected]
Search for more papers by this authorPeiyu Wang
Thoracic Oncology Institute/Department of Thoracic Surgery, Peking University People's Hospital, Beijing, China
Search for more papers by this authorShaodong Wang
Thoracic Oncology Institute/Department of Thoracic Surgery, Peking University People's Hospital, Beijing, China
Search for more papers by this authorZewen Sun
Thoracic Oncology Institute/Department of Thoracic Surgery, Peking University People's Hospital, Beijing, China
Search for more papers by this authorHao Li
Thoracic Oncology Institute/Department of Thoracic Surgery, Peking University People's Hospital, Beijing, China
Search for more papers by this authorYaxing Zhao
Infinity Scope Biotechnology Co. Ltd., Hangzhou, China
Search for more papers by this authorYun Li
Thoracic Oncology Institute/Department of Thoracic Surgery, Peking University People's Hospital, Beijing, China
Search for more papers by this authorFan Yang
Thoracic Oncology Institute/Department of Thoracic Surgery, Peking University People's Hospital, Beijing, China
Search for more papers by this authorJun Wang
Thoracic Oncology Institute/Department of Thoracic Surgery, Peking University People's Hospital, Beijing, China
Search for more papers by this authorKezhong Chen
Thoracic Oncology Institute/Department of Thoracic Surgery, Peking University People's Hospital, Beijing, China
Search for more papers by this authorCorresponding Author
Mantang Qiu
Thoracic Oncology Institute/Department of Thoracic Surgery, Peking University People's Hospital, Beijing, China
Correspondence
Mantang Qiu and Xiao Li, Thoracic Oncology Institute/Department of Thoracic Surgery, Peking University People's Hospital, 11 Xizhimen South Street, Beijing 100044, China.
Email: [email protected], [email protected], and [email protected], [email protected]
Search for more papers by this authorCorresponding Author
Xiao Li
Thoracic Oncology Institute/Department of Thoracic Surgery, Peking University People's Hospital, Beijing, China
Correspondence
Mantang Qiu and Xiao Li, Thoracic Oncology Institute/Department of Thoracic Surgery, Peking University People's Hospital, 11 Xizhimen South Street, Beijing 100044, China.
Email: [email protected], [email protected], and [email protected], [email protected]
Search for more papers by this authorAbstract
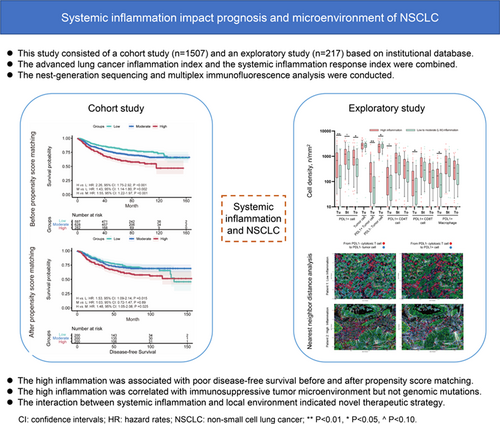
The impact of host condition on prognosis of non-small cell lung cancer (NSCLC) and the interaction between host and NSCLC remain unclear. This study investigated the association between systemic inflammation and prognosis and characteristics of radically resected NSCLC. This study consisted of a cohort study and an exploratory study of institutional prospective databases. All participants underwent video-assisted thoracoscopic lobectomy as the primary treatment. Systemic inflammation was assessed before surgery using the advanced lung cancer inflammation index and the systemic inflammation response index. Next-generation sequencing and multiplex immunofluorescence analysis were conducted to delineate tumor characteristics. In the cohort study including 1507 participants, high inflammation was associated with poor disease-free survival and overall survival before and after propensity score matching and in multivariable analysis. Systemic inflammation showed good prognostic value for stage IA-IB NSCLC, and the prognostic value diminished with upstaging of NSCLC. In the exploratory study including 217 adenocarcinomas, tumor microenvironment of high inflammation group showed a greater abundance of PDL1+ tumor cells and immune cells, which were independent from driver gene mutations and clinicopathological characteristics. Spatial analysis demonstrated a higher frequency of immune-suppressed cellular neighborhood, increased avoidance between immune cells and PDL1- tumor cells and compromised immune killing and presentation in tumor microenvironment of high inflammation group. Systemic inflammation showed limited association with genomic mutations. Systemic inflammation may influence the prognosis of NSCLC at both the systematic level and the local immune response. The correlation between high inflammation and immunosuppressive microenvironment indicates a novel thread for anticancer treatment.
Graphical Abstract
What's new?
The role of host systemic inflammation in radically-resected non-small cell lung cancer (NSCLC) remains unclear. This study demonstrated baseline systemic inflammation as an independent prognostic factor for early-stage NSCLC after radical resection, with high inflammation being associated with poor disease-free and overall survival. High inflammation also correlated with an immunosuppressive tumor microenvironment, but not with genomic mutations. Systemic inflammation may influence the prognosis of NSCLC not only at the systemic level but also by modulating the local immune response. The revealed interactions between host systemic inflammation and the tumor microenvironment may indicate a novel avenue for anticancer therapy in NSCLC.
CONFLICT OF INTEREST STATEMENT
The authors declare that they have no conflict of interest.
Open Research
DATA AVAILABILITY STATEMENT
The data that support the findings of this study are available from the corresponding author upon reasonable request.
Supporting Information
| Filename | Description |
|---|---|
| ijc34547-sup-0001-Tables.xlsxExcel 2007 spreadsheet , 40 KB | Table S1. Broad-panel NGS gene. Table S2. The sequencing coverage and quality statistics of each sample. |
| ijc34547-sup-0002-Supinfo.pdfPDF document, 1.2 MB | Data S1. Supporting Information. |
Please note: The publisher is not responsible for the content or functionality of any supporting information supplied by the authors. Any queries (other than missing content) should be directed to the corresponding author for the article.
REFERENCES
- 1 Global Burden of Disease 2019 Cancer Collaboration. Cancer incidence, mortality, years of life lost, years lived with disability, and disability-adjusted life years for 29 cancer groups from 2010 to 2019: a systematic analysis for the global burden of disease study 2019. JAMA Oncol. 2022; 8(3): 420-444. doi:10.1001/jamaoncol.2021.6987
- 2Tseng CH, Tsuang BJ, Chiang CJ, et al. The relationship between air pollution and lung cancer in nonsmokers in Taiwan. J Thorac Oncol. 2019; 14(5): 784-792. doi:10.1016/j.jtho.2018.12.033
- 3Pennathur A, Brunelli A, Criner GJ, et al. Definition and assessment of high risk in patients considered for lobectomy for stage I non-small cell lung cancer: the American Association for Thoracic Surgery expert panel consensus document. J Thorac Cardiovasc Surg. 2021; 162(6): 1605-1618. doi:10.1016/j.jtcvs.2021.07.030
- 4Chen K, Wang X, Yang F, et al. Propensity-matched comparison of video-assisted thoracoscopic with thoracotomy lobectomy for locally advanced non-small cell lung cancer. J Thorac Cardiovasc Surg. 2017; 153(4): 967-976. doi:10.1016/j.jtcvs.2016.12.008
- 5Tan AC, Tan DSW. Targeted therapies for lung cancer patients with oncogenic driver molecular alterations. J Clin Oncol. 2022; 40(6): 611-625. doi:10.1200/JCO.21.01626
- 6Yu H, Boyle TA, Zhou C, Rimm DL, Hirsch FR. PD-L1 expression in lung cancer. J Thorac Oncol. 2016; 11(7): 964-975. doi:10.1016/j.jtho.2016.04.014
- 7Mao W, Wang K, Wu Y, et al. Prognostic significance of modified advanced lung cancer inflammation index in patients with renal cell carcinoma undergoing laparoscopic nephrectomy: a multi-institutional, propensity score matching cohort study. Front Nutr. 2021; 8:781647. doi:10.3389/fnut.2021.781647
- 8Dolan RD, McSorley ST, Park JH, et al. The prognostic value of systemic inflammation in patients undergoing surgery for colon cancer: comparison of composite ratios and cumulative scores. Br J Cancer. 2018; 119(1): 40-51. doi:10.1038/s41416-018-0095-9
- 9Yin C, Toiyama Y, Okugawa Y, et al. Clinical significance of advanced lung cancer inflammation index, a nutritional and inflammation index, in gastric cancer patients after surgical resection: a propensity score matching analysis. Clin Nutr. 2021; 40(3): 1130-1136. doi:10.1016/j.clnu.2020.07.018
- 10Song M, Zhang Q, Song C, et al. The advanced lung cancer inflammation index is the optimal inflammatory biomarker of overall survival in patients with lung cancer. J Cachexia Sarcopenia Muscle. 2022; 13(5): 2504-2514. doi:10.1002/jcsm.13032
- 11Qi WX, Xiang Y, Zhao S, Chen J. Assessment of systematic inflammatory and nutritional indexes in extensive-stage small-cell lung cancer treated with first-line chemotherapy and atezolizumab. Cancer Immunol Immunother. 2021; 70(11): 3199-3206. doi:10.1007/s00262-021-02926-3
- 12Mountzios G, Samantas E, Senghas K, et al. Association of the advanced lung cancer inflammation index (ALI) with immune checkpoint inhibitor efficacy in patients with advanced non-small-cell lung cancer. ESMO Open. 2021; 6(5):100254. doi:10.1016/j.esmoop.2021.100254
- 13Yang H, Wang K, Li B, Li S, Li Y, Yuan L. The prognostic role of blood inflammatory biomarkers and EGFR mutation status in stage IIIA/N2 non-small cell lung cancer patients treated with Trimodality therapy. Front Oncol. 2021; 11:707041. doi:10.3389/fonc.2021.707041
- 14Qi Q, Zhuang L, Shen Y, et al. A novel systemic inflammation response index (SIRI) for predicting the survival of patients with pancreatic cancer after chemotherapy. Cancer. 2016; 122(14): 2158-2167. doi:10.1002/cncr.30057
- 15Wang X, Ni Q, Wang J, Wu S, Chen P, Xing D. Systemic inflammation response index is a promising prognostic marker in elderly patients with heart failure: a retrospective cohort study. Front Cardiovasc Med. 2022; 9:871031. doi:10.3389/fcvm.2022.871031
- 16Barth DA, Brenner C, Riedl JM, et al. External validation of the prognostic relevance of the advanced lung cancer inflammation index (ALI) in pancreatic cancer patients. Cancer Med. 2020; 9(15): 5473-5479. doi:10.1002/cam4.3233
- 17Ren L, Xu J, Li J, et al. A prognostic model incorporating inflammatory cells and cytokines for newly diagnosed multiple myeloma patients. Clin Exp Med. 2023. Epub ahead of print. doi:10.1007/s10238-023-00992-8
- 18von Elm E, Altman DG, Egger M, Pocock SJ, Gøtzsche PC, Vandenbroucke JP. The strengthening the reporting of observational studies in epidemiology (STROBE) statement: guidelines for reporting observational studies. Lancet. 2007; 370(9596): 1453-1457. doi:10.1016/s0140-6736(07)61602-x
- 19Sullivan R, Alatise OI, Anderson BO, et al. Global cancer surgery: delivering safe, affordable, and timely cancer surgery. Lancet Oncol. 2015; 16(11): 1193-1224. doi:10.1016/s1470-2045(15)00223-5
- 20Camp RL, Dolled-Filhart M, Rimm DL. X-tile: a new bio-informatics tool for biomarker assessment and outcome-based cut-point optimization. Clin Cancer Res. 2004; 10(21): 7252-7259. doi:10.1158/1078-0432.CCR-04-0713
- 21Li H, Sun Z, Li Y, et al. Disparate genomic characteristics of patients with early-stage lung adenocarcinoma manifesting as radiological subsolid or solid lesions. Lung Cancer. 2022; 166: 178-188. doi:10.1016/j.lungcan.2022.02.012
- 22Huang L, Jiang X-L, Liang H-B, et al. Genetic profiling of primary and secondary tumors from patients with lung adenocarcinoma and bone metastases reveals targeted therapy options. Mol Med. 2020; 26(1): 88. doi:10.1186/s10020-020-00197-9
- 23Peng H, Wu X, Liu S, et al. Multiplex immunofluorescence and single-cell transcriptomic profiling reveal the spatial cell interaction networks in the non-small cell lung cancer microenvironment. Clin Transl Med. 2023; 13(1):e1155. doi:10.1002/ctm2.1155
- 24Zheng X, Weigert A, Reu S, et al. Spatial density and distribution of tumor-associated macrophages predict survival in non-small cell lung carcinoma. Cancer Res. 2020; 80(20): 4414-4425. doi:10.1158/0008-5472.CAN-20-0069
- 25Wu F, Fan J, He Y, et al. Single-cell profiling of tumor heterogeneity and the microenvironment in advanced non-small cell lung cancer. Nat Commun. 2021; 12(1): 2540. doi:10.1038/s41467-021-22801-0
- 26Carstens JL, Correa de Sampaio P, Yang D, et al. Spatial computation of intratumoral T cells correlates with survival of patients with pancreatic cancer. Nat Commun. 2017; 8:15095. doi:10.1038/ncomms15095
- 27Yoon HH, Jin Z, Kour O, et al. Association of PD-L1 expression and other variables with benefit from immune checkpoint inhibition in advanced gastroesophageal cancer: systematic review and meta-analysis of 17 phase 3 randomized clinical trials. JAMA Oncol. 2022; 8(10): 1456-1465. doi:10.1001/jamaoncol.2022.3707
- 28Schürch CM, Bhate SS, Barlow GL, et al. Coordinated cellular neighborhoods orchestrate antitumoral immunity at the colorectal cancer invasive front. Cell. 2020; 182(5): 1341-1359. doi:10.1016/j.cell.2020.07.005
- 29Sheng J, Zhang J, Wang L, et al. Topological analysis of hepatocellular carcinoma tumour microenvironment based on imaging mass cytometry reveals cellular neighbourhood regulated reversely by macrophages with different ontogeny. Gut. 2022; 71(6): 1176-1191. doi:10.1136/gutjnl-2021-324339
- 30Yang L, Zhang W, Sun J, et al. Functional status and spatial interaction of T cell subsets driven by specific tumor microenvironment correlate with recurrence of non-small cell lung cancer. Front Immunol. 2022; 13:1022638. doi:10.3389/fimmu.2022.1022638
- 31Grivennikov SI, Greten FR, Karin M. Immunity, inflammation, and cancer. Cell. 2010; 140(6): 883-899. doi:10.1016/j.cell.2010.01.025
- 32Gale D, Heider K, Ruiz-Valdepenas A, et al. Residual ctDNA after treatment predicts early relapse in patients with early-stage non-small cell lung cancer. Ann Oncol. 2022; 33(5): 500-510. doi:10.1016/j.annonc.2022.02.007
- 33Elinav E, Nowarski R, Thaiss CA, Hu B, Jin C, Flavell RA. Inflammation-induced cancer: crosstalk between tumours, immune cells and microorganisms. Nat Rev Cancer. 2013; 13(11): 759-771. doi:10.1038/nrc3611
- 34Sharpe AH, Wherry EJ, Ahmed R, Freeman GJ. The function of programmed cell death 1 and its ligands in regulating autoimmunity and infection. Nat Immunol. 2007; 8(3): 239-245. doi:10.1038/ni1443
- 35Ishida Y, Agata Y, Shibahara K, Honjo T. Induced expression of PD-1, a novel member of the immunoglobulin gene superfamily, upon programmed cell death. EMBO J. 1992; 11(11): 3887-3895. doi:10.1002/j.1460-2075.1992.tb05481.x
- 36Zak KM, Kitel R, Przetocka S, et al. Structure of the complex of human programmed death 1, PD-1, and its ligand PD-L1. Structure. 2015; 23(12): 2341-2348. doi:10.1016/j.str.2015.09.010
- 37Sun C, Mezzadra R, Schumacher TN. Regulation and function of the PD-L1 checkpoint. Immunity. 2018; 48(3): 434-452. doi:10.1016/j.immuni.2018.03.014
- 38Spranger S, Spaapen RM, Zha Y, et al. Up-regulation of PD-L1, IDO, and T(regs) in the melanoma tumor microenvironment is driven by CD8(+) T cells. Sci Transl Med. 2013; 28(5):200ra116. doi:10.1126/scitranslmed.3006504
- 39Park JJ, Omiya R, Matsumura Y, et al. B7-H1/CD80 interaction is required for the induction and maintenance of peripheral T-cell tolerance. Blood. 2010; 116(8): 1291-1298. doi:10.1182/blood-2010-01-265975
- 40Erickson JJ, Gilchuk P, Hastings AK, et al. Viral acute lower respiratory infections impair CD8+ T cells through PD-1. J Clin Investig. 2012; 122(8): 2967-2982. doi:10.1172/jci62860
- 41Yao S, Wang S, Zhu Y, et al. PD-1 on dendritic cells impedes innate immunity against bacterial infection. Blood. 2009; 113(23): 5811-5818. doi:10.1182/blood-2009-02-203141
- 42Herbst RS, Soria J-C, Kowanetz M, et al. Predictive correlates of response to the anti-PD-L1 antibody MPDL3280A in cancer patients. Nature. 2014; 515(7528): 563-567. doi:10.1038/nature14011
- 43Fehrenbacher L, Spira A, Ballinger M, et al. Atezolizumab versus docetaxel for patients with previously treated non-small-cell lung cancer (POPLAR): a multicentre, open-label, phase 2 randomised controlled trial. Lancet. 2016; 387(10030): 1837-1846. doi:10.1016/s0140-6736(16)00587-0
- 44Tsakiroglou AM, Fergie M, Oguejiofor K, et al. Spatial proximity between T and PD-L1 expressing cells as a prognostic biomarker for oropharyngeal squamous cell carcinoma. Br J Cancer. 2019; 122(4): 539-544. doi:10.1038/s41416-019-0634-z
- 45Køstner AH, Nielsen PS, Georgsen JB, et al. Systemic inflammation associates with a myeloid inflamed tumor microenvironment in primary resected colon cancer—may cold tumors simply be too hot? Front Immunol. 2021; 12: 12. doi:10.3389/fimmu.2021.716342
- 46Choi Y, Kim JW, Nam KH, et al. Systemic inflammation is associated with the density of immune cells in the tumor microenvironment of gastric cancer. Gastric Cancer. 2016; 20(4): 602-611. doi:10.1007/s10120-016-0642-0
- 47Stares M, Ding TE, Stratton C, et al. Biomarkers of systemic inflammation predict survival with first-line immune checkpoint inhibitors in non-small-cell lung cancer. ESMO Open. 2022; 7(2):100445. doi:10.1016/j.esmoop.2022.100445
- 48Budczies J, Kirchner M, Kluck K, et al. Deciphering the immunosuppressive tumor microenvironment in ALK- and EGFR-positive lung adenocarcinoma. Cancer Immunol Immunother. 2021; 71(2): 251-265. doi:10.1007/s00262-021-02981-w
- 49Gainor JF, Shaw AT, Sequist LV, et al. EGFR mutations and ALK rearrangements are associated with low response rates to PD-1 pathway blockade in non-small cell lung cancer: a retrospective analysis. Clin Cancer Res. 2016; 22(18): 4585-4593. doi:10.1158/1078-0432.ccr-15-3101
- 50Hu H, Cheng R, Wang Y, et al. Oncogenic KRAS signaling drives evasion of innate immune surveillance in lung adenocarcinoma by activating CD47. J Clin Invest. 2023; 133(2):e153470. doi:10.1172/JCI153470
- 51Calvani R, Marini F, Cesari M, et al. Systemic inflammation, body composition, and physical performance in old community-dwellers. J Cachexia Sarcopenia Muscle. 2017; 8(1): 69-77. doi:10.1002/jcsm.12134
- 52Roubenoff R. Physical activity, inflammation, and muscle loss. Nutr Rev. 2007; 65(12): 208-212. doi:10.1301/nr.2007.dec.S208-S212
- 53Zitvogel L, Pietrocola F, Kroemer G. Nutrition, inflammation and cancer. Nat Immunol. 2017; 18(8): 843-850. doi:10.1038/ni.3754
- 54Crusz SM, Balkwill FR. Inflammation and cancer: advances and new agents. Nat Rev Clin Oncol. 2015; 12(10): 584-596. doi:10.1038/nrclinonc.2015.105