Proxalutamide in metastatic castration-resistant prostate cancer: Primary analysis of a multicenter, randomized, open-label, phase 2 trial
Tie Zhou and Shengfei Qin contributed equally to our study.
Abstract
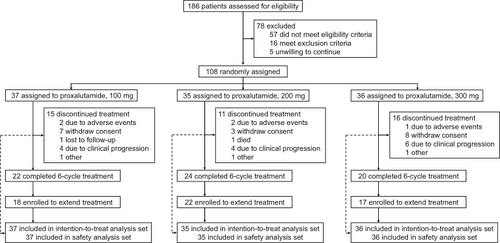
We aim to assess the safety and efficacy of proxalutamide, a novel androgen receptor antagonist, for men with metastatic castration-resistant prostate cancer (mCRPC) in a multicenter, randomized, open-label, phase 2 trial. In our study, the enrolled mCRPC patients were randomized to 100, 200 and 300 mg dose groups at 1:1:1. The primary efficacy endpoint was prostate-specific antigen (PSA) response rate. The secondary endpoints included objective response rate (ORR), disease control rate (DCR) and time to PSA and radiographic progression. Safety and pharmacokinetics were also assessed. Finally, there were 108 patients from 17 centers being enrolled. By week 16, there were 13 (35.1%), 12 (36.4%) and 15 (42.9%) patients with confirmed 50% or greater PSA decline in 100 mg (n = 37), 200 mg (n = 33) and 300 mg (n = 35) groups, respectively. Among the 19 patients with target lesions at study entry, three (15.8%) had a partial response and 12 (63.2%) had stable disease. The ORRs of 20.0%, 22.2%, 0% and DCRs of 80.0%, 88.9%, 60.0% were, respectively, achieved in 100, 200 and 300 mg groups. By the maximum follow-up time of 24 weeks, there were 42.6% and 10.2% of cases experiencing PSA progression and radiographic progression, respectively. Overall, adverse events (AEs) were experienced by 94.4% of patients, most of which were mild or moderate. There were 28 patients experiencing ≥grade 3 AEs. The most common AEs were fatigue (17.6%), anemia (14.8%), elevated AST (14.8%) and ALT (13.0%), decreased appetite (13.0%). These findings preliminarily showed the promising antitumor activity of proxalutamide in patients with mCRPC with a manageable safety profile. The proxalutamide dose of 200 mg daily is recommended for future phase 3 trial (Clinical trial registration no. CTR20170177).
Graphical Abstract
What's new?
New options are needed for patients with metastatic castration-resistant prostate cancer (mCRPC). Here, the authors present the results of a phase 2, open-label trial of a new androgen receptor antagonist, proxalutamide. Patients were randomized to one of three doses and the primary endpoint was PSA level. Across all doses, around 40% of patients had a PSA decline of 50% or more, and the drug was judged to be safe and well-tolerated.
CONFLICT OF INTEREST STATEMENT
All authors were not employee of Kintor Pharmaceutical Inc., Suzhou, China, and declared no potential conflicts of interest.
Open Research
DATA AVAILABILITY STATEMENT
The data that support the findings of our study are available on request from the corresponding author.