Cancer stem cell-immune cell collusion in immunotherapy
Yuan-Yuan Wang
The State Key Laboratory Breeding Base of Basic Science of Stomatology (Hubei-MOST) & Key Laboratory of Oral Biomedicine, Ministry of Education, School and Hospital of Stomatology, Wuhan University, Wuhan, Hubei, People's Republic of China
Search for more papers by this authorWen-Da Wang
The State Key Laboratory Breeding Base of Basic Science of Stomatology (Hubei-MOST) & Key Laboratory of Oral Biomedicine, Ministry of Education, School and Hospital of Stomatology, Wuhan University, Wuhan, Hubei, People's Republic of China
Search for more papers by this authorCorresponding Author
Zhi-Jun Sun
The State Key Laboratory Breeding Base of Basic Science of Stomatology (Hubei-MOST) & Key Laboratory of Oral Biomedicine, Ministry of Education, School and Hospital of Stomatology, Wuhan University, Wuhan, Hubei, People's Republic of China
Department of Oral Maxillofacial-Head Neck Oncology, School and Hospital of Stomatology, Wuhan University, Wuhan, Hubei, People's Republic of China
Correspondence
Zhi-Jun Sun, The State Key Laboratory Breeding Base of Basic Science of Stomatology (Hubei-MOST), Key Laboratory of Oral Biomedicine Ministry of Education, Department of Oral Maxillofacial-Head Neck Oncology, School and Hospital of Stomatology, Wuhan University, 237 Luoyu Road, Wuhan 430079, Hubei, People's Republic of China.
Email: [email protected]
Search for more papers by this authorYuan-Yuan Wang
The State Key Laboratory Breeding Base of Basic Science of Stomatology (Hubei-MOST) & Key Laboratory of Oral Biomedicine, Ministry of Education, School and Hospital of Stomatology, Wuhan University, Wuhan, Hubei, People's Republic of China
Search for more papers by this authorWen-Da Wang
The State Key Laboratory Breeding Base of Basic Science of Stomatology (Hubei-MOST) & Key Laboratory of Oral Biomedicine, Ministry of Education, School and Hospital of Stomatology, Wuhan University, Wuhan, Hubei, People's Republic of China
Search for more papers by this authorCorresponding Author
Zhi-Jun Sun
The State Key Laboratory Breeding Base of Basic Science of Stomatology (Hubei-MOST) & Key Laboratory of Oral Biomedicine, Ministry of Education, School and Hospital of Stomatology, Wuhan University, Wuhan, Hubei, People's Republic of China
Department of Oral Maxillofacial-Head Neck Oncology, School and Hospital of Stomatology, Wuhan University, Wuhan, Hubei, People's Republic of China
Correspondence
Zhi-Jun Sun, The State Key Laboratory Breeding Base of Basic Science of Stomatology (Hubei-MOST), Key Laboratory of Oral Biomedicine Ministry of Education, Department of Oral Maxillofacial-Head Neck Oncology, School and Hospital of Stomatology, Wuhan University, 237 Luoyu Road, Wuhan 430079, Hubei, People's Republic of China.
Email: [email protected]
Search for more papers by this authorYuan-Yuan Wang and Wen-Da Wang contributed equally to this study.
Abstract
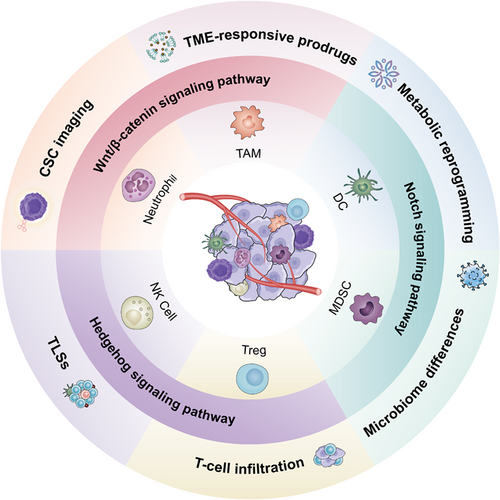
Immunotherapy has pioneered a new era of tumor treatment, in which the immune checkpoint blockade (ICB) exerts significant superiority in overcoming tumor immune escape. However, the formation of an immune-suppressive tumor microenvironment (TME) and the lack of effective activation of the immune response have become major obstacles limiting its development. Emerging reports indicate that cancer stem cells (CSCs) potentially play important roles in treatment resistance and progressive relapse, while current research is usually focused on CSCs themselves. In this review, we mainly emphasize the collusions between CSCs and tumor-infiltrating immune cells. We focus on the summary of CSC-immune cell crosstalk signaling pathways in ICB resistance and highlight the application of targeted drugs to improve the ICB response.
Graphical Abstract
CONFLICT OF INTEREST
The authors declare no conflicts of interest.
REFERENCES
- 1Bayik D, Lathia JD. Cancer stem cell-immune cell crosstalk in tumour progression. Nat Rev Cancer. 2021; 21(8): 526-536.
- 2Chen P, Hsu WH, Han J, Xia Y, DePinho RA. Cancer stemness meets immunity: from mechanism to therapy. Cell Rep. 2021; 34(1):108597.
- 3Huang T, Song X, Xu D, et al. Stem cell programs in cancer initiation, progression, and therapy resistance. Theranostics. 2020; 10(19): 8721-8743.
- 4Yang L, Li A, Lei Q, Zhang Y. Tumor-intrinsic signaling pathways: key roles in the regulation of the immunosuppressive tumor microenvironment. J Hematol Oncol. 2019; 12(1): 125.
- 5Sun R, Zhang Z, Bao R, et al. Loss of SIRT5 promotes bile acid-induced immunosuppressive microenvironment and hepatocarcinogenesis. J Hepatol. 2022; 77(2): 453-466.
- 6Dong F, Qin X, Wang B, et al. ALKBH5 facilitates hypoxia-induced paraspeckle assembly and IL8 secretion to generate an immunosuppressive tumor microenvironment. Cancer Res. 2021; 81(23): 5876-5888.
- 7Zhou G, Sprengers D, Mancham S, et al. Reduction of immunosuppressive tumor microenvironment in cholangiocarcinoma by ex vivo targeting immune checkpoint molecules. J Hepatol. 2019; 71(4): 753-762.
- 8Topalian SL, Taube JM, Pardoll DM. Neoadjuvant checkpoint blockade for cancer immunotherapy. Science. 2020; 367(6477): eaax0182.
- 9Jin Z, Sinicrope FA. Mismatch repair-deficient colorectal cancer: building on checkpoint blockade. J Clin Oncol. 2022; 40(24): 2735-2750.
- 10Vlachostergios PJ, Faltas BM. Treatment resistance in urothelial carcinoma: an evolutionary perspective. Nat Rev Clin Oncol. 2018; 15(8): 495-509.
- 11van der Merwe M, van Niekerk G, Fourie C, du Plessis M, Engelbrecht AM. The impact of mitochondria on cance treatment resistance. Cell Oncol (Dordr). 2021; 44(5): 983-995.
- 12Błach J, Wojas-Krawczyk K, Nicoś M, et al. Failure of immunotherapy-the molecular and immunological origin of immunotherapy resistance in Lung cancer. Int J Mol Sci. 2021; 22(16): 9030.
- 13Ascierto PA, Butterfield LH, Demaria S, et al. The great debate at “Immunotherapy Bridge 2018,” Naples, November 29th, 2018. J Immunother Cancer. 2019; 7(1): 221.
- 14Nishino M, Ramaiya NH, Hatabu H, Hodi FS. Monitoring immune-checkpoint blockade: response evaluation and biomarker development. Nat Rev Clin Oncol. 2017; 14(11): 655-668.
- 15Korman AJ, Garrett-Thomson SC, Lonberg N. The foundations of immune checkpoint blockade and the ipilimumab approval decennial. Nat Rev Drug Discov. 2022; 21(7): 509-528.
- 16Morad G, Helmink BA, Sharma P, Wargo JA. Hallmarks of response, resistance, and toxicity to immune checkpoint blockade. Cell. 2021; 184(21): 5309-5337.
- 17Sharma P, Hu-Lieskovan S, Wargo JA, Ribas A. Primary, adaptive, and acquired resistance to cancer immunotherapy. Cell. 2017; 168(4): 707-723.
- 18Lambert AW, Weinberg RA. Linking EMT programmes to normal and neoplastic epithelial stem cells. Nat Rev Cancer. 2021; 21(5): 325-338.
- 19Prince ME, Ailles LE. Cancer stem cells in head and neck squamous cell cancer. J Clin Oncol. 2008; 26(17): 2871-2875.
- 20Saba JA, Liakath-Ali K, Green R, Watt FM. Translational control of stem cell function. Nat Rev Mol Cell Biol. 2021; 22(10): 671-690.
- 21Raghavan S, Mehta P, Ward MR, et al. Personalized medicine-based approach to model patterns of chemoresistance and tumor recurrence using ovarian cancer stem cell spheroids. Clin Cancer Res. 2017; 23(22): 6934-6945.
- 22Brugnoli F, Grassilli S, Piazzi M, et al. In triple negative breast tumor cells, PLC-β2 promotes the conversion of CD133high to CD133low phenotype and reduces the CD133-related invasiveness. Mol Cancer. 2013; 12: 165.
- 23Chen X, Lingala S, Khoobyari S, Nolta J, Zern MA, Wu J. Epithelial mesenchymal transition and hedgehog signaling activation are associated with chemoresistance and invasion of hepatoma subpopulations. J Hepatol. 2011; 55(4): 838-845.
- 24Lu J, Ye X, Fan F, et al. Endothelial cells promote the colorectal cancer stem cell phenotype through a soluble form of Jagged-1. Cancer Cell. 2013; 23(2): 171-185.
- 25Zhang R, Tu J, Liu S. Novel molecular regulators of breast cancer stem cell plasticity and heterogeneity. Semin Cancer Biol. 2022; 82: 11-25.
- 26Lindahl R. Identification of hepatocarcinogenesis-associated aldehyde dehydrogenase in normal rat urinary bladder. Cancer Res. 1986; 46(5): 2502-2506.
- 27Emmink BL, Van Houdt WJ, Vries RG, et al. Differentiated human colorectal cancer cells protect tumor-initiating cells from irinotecan. Gastroenterology. 2011; 141(1): 269-278.
- 28Jia L, Zhang W, Wang CY. BMI1 inhibition eliminates residual cancer stem cells after PD1 blockade and activates antitumor immunity to prevent metastasis and relapse. Cell Stem Cell. 2020; 27(2): 238-253 e6.
- 29Wang C, Li Y, Jia L, et al. CD276 expression enables squamous cell carcinoma stem cells to evade immune surveillance. Cell Stem Cell. 2021; 28(9): 1597-1613.
- 30Chen D, Zhang W, Jia L, Wang C, Wang CY. Generation of a squamous cell carcinoma mouse model for lineage tracing of BMI1+ cancer stem cells. STAR Protoc. 2021; 2(2):100484.
- 31Mikkilineni L, Kochenderfer JN. CAR T cell therapies for patients with multiple myeloma. Nat Rev Clin Oncol. 2021; 18(2): 71-84.
- 32Amini L, Silbert SK, Maude SL, et al. Preparing for CAR T cell therapy: patient selection, bridging therapies and lymphodepletion. Nat Rev Clin Oncol. 2022; 19(5): 342-355.
- 33Singh AK, McGuirk JP. CAR T cells: continuation in a revolution of immunotherapy. Lancet Oncol. 2020; 21(3): e168-e178.
- 34Davies LC, Jenkins SJ, Allen JE, Taylor PR. Tissue-resident macrophages. Nat Immunol. 2013; 14(10): 986-995.
- 35Li H, Xiao Y, Li Q, et al. The allergy mediator histamine confers resistance to immunotherapy in cancer patients via activation of the macrophage histamine receptor H1. Cancer Cell. 2022; 40(1): 36-52.
- 36Huang H, Wang C, Liu F, et al. Reciprocal network between cancer stem-like cells and macrophages facilitates the progression and androgen deprivation therapy resistance of prostate cancer. Clin Cancer Res. 2018; 24(18): 4612-4626.
- 37Li X, Bu W, Meng L, et al. CXCL12/CXCR4 pathway orchestrates CSC-like properties by CAF recruited tumor associated macrophage in OSCC. Exp Cell Res. 2019; 378(2): 131-138.
- 38Yang L, Shi P, Zhao G, et al. Targeting cancer stem cell pathways for cancer therapy. Signal Transduct Target Ther. 2020; 5(1): 8.
- 39Gomez KE, Wu F, Keysar SB, et al. Cancer cell CD44 mediates macrophage/monocyte-driven regulation of head and neck cancer stem cells. Cancer Res. 2020; 80(19): 4185-4198.
- 40Raghavan S, Mehta P, Xie Y, Lei YL, Mehta G. Ovarian cancer stem cells and macrophages reciprocally interact through the WNT pathway to promote pro-tumoral and malignant phenotypes in 3D engineered microenvironments. J Immunother Cancer. 2019; 7(1): 190.
- 41Wan S, Zhao E, Kryczek I, et al. Tumor-associated macrophages produce interleukin 6 and signal via STAT3 to promote expansion of human hepatocellular carcinoma stem cells. Gastroenterology. 2014; 147(6): 1393-1404.
- 42Lu CS, Shiau AL, Su BH, et al. Oct4 promotes M2 macrophage polarization through upregulation of macrophage colony-stimulating factor in lung cancer. J Hematol Oncol. 2020; 13(1): 62.
- 43Nusblat LM, Carroll MJ, Roth CM. Crosstalk between M2 macrophages and glioma stem cells. Cell Oncol (Dordr). 2017; 40(5): 471-482.
- 44den Dekker E, Grefte S, Huijs T, et al. Monocyte cell surface glycosaminoglycans positively modulate IL-4-induced differentiation toward dendritic cells. J Immunol. 2008; 180(6): 3680-3688.
- 45Cytlak U, Resteu A, Pagan S, et al. Differential IRF8 transcription factor requirement defines two pathways of dendritic cell development in humans. Immunity. 2020; 53(2): 353-370.
- 46Conrad C, Gregorio J, Wang YH, et al. Plasmacytoid dendritic cells promote immunosuppression in ovarian cancer via ICOS costimulation of Foxp3(+) T-regulatory cells. Cancer Res. 2012; 72(20): 5240-5249.
- 47Chauhan D, Singh AV, Brahmandam M, et al. Functional interaction of plasmacytoid dendritic cells with multiple myeloma cells: a therapeutic target. Cancer Cell. 2009; 16(4): 309-323.
- 48Dashti A, Ebrahimi M, Hadjati J, Memarnejadian A, Moazzeni SM. Dendritic cell based immunotherapy using tumor stem cells mediates potent antitumor immune responses. Cancer Lett. 2016; 374(1): 175-185.
- 49El-Ashmawy NE, El-Zamarany EA, Salem ML, et al. A new strategy for enhancing antitumor immune response using dendritic cells loaded with chemo-resistant cancer stem-like cells in experimental mice model. Mol Immunol. 2019; 111: 106-117.
- 50Lu L, Tao H, Chang AE, et al. Cancer stem cell vaccine inhibits metastases of primary tumors and induces humoral immune responses against cancer stem cells. Onco Targets Ther. 2015; 4(3):e990767.
- 51Hu Y, Lu L, Xia Y, et al. Therapeutic efficacy of cancer stem cell vaccines in the adjuvant setting. Cancer Res. 2016; 76(16): 4661-4672.
- 52Ruan S, Lin M, Zhu Y, et al. Integrin β4-targeted cancer immunotherapies inhibit tumor growth and decrease metastasis. Cancer Res. 2020; 80(4): 771-783.
- 53Gabrilovich DI, Nagaraj S. Myeloid-derived suppressor cells as regulators of the immune system. Nat Rev Immunol. 2009; 9(3): 162-174.
- 54Talmadge JE, Gabrilovich DI. History of myeloid-derived suppressor cells. Nat Rev Cancer. 2013; 13(10): 739-752.
- 55Shidal C, Singh NP, Nagarkatti P, Nagarkatti M. MicroRNA-92 expression in CD133 melanoma stem cells regulates immunosuppression in the tumor microenvironment via integrin-dependent activation of TGFβ. Cancer Res. 2019; 79(14): 3622-3635.
- 56Chikamatsu K, Takahashi G, Sakakura K, Ferrone S, Masuyama K. Immunoregulatory properties of CD44+ cancer stem-like cells in squamous cell carcinoma of the head and neck. Head Neck. 2011; 33(2): 208-215.
- 57Liu C, Qiang J, Deng Q, et al. ALDH1A1 activity in tumor-initiating cells remodels myeloid-derived suppressor cells to promote breast cancer progression. Cancer Res. 2021; 81(23): 5919-5934.
- 58Cui TX, Kryczek I, Zhao L, et al. Myeloid-derived suppressor cells enhance stemness of cancer cells by inducing microRNA101 and suppressing the corepressor CtBP2. Immunity. 2013; 39(3): 611-621.
- 59Ouzounova M, Lee E, Piranlioglu R, et al. Monocytic and granulocytic myeloid derived suppressor cells differentially regulate spatiotemporal tumour plasticity during metastatic cascade. Nat Commun. 2017; 8:14979.
- 60Peng D, Tanikawa T, Li W, et al. Myeloid-derived suppressor cells endow stem-like qualities to breast cancer cells through IL6/STAT3 and NO/NOTCH cross-talk signaling. Cancer Res. 2016; 76(11): 3156-3165.
- 61Komura N, Mabuchi S, Shimura K, et al. The role of myeloid-derived suppressor cells in increasing cancer stem-like cells and promoting PD-L1 expression in epithelial ovarian cancer. Cancer Immunol Immunother. 2020; 69(12): 2477-2499.
- 62Li X, Wang J, Wu W, et al. Myeloid-derived suppressor cells promote epithelial ovarian cancer cell stemness by inducing the CSF2/p-STAT3 signalling pathway. FEBS J. 2020; 287(23): 5218-5235.
- 63Xie M, Wei J, Xu J. Inducers, attractors and modulators of CD4 Treg cells in non-small-cell lung cancer. Front Immunol. 2020; 11: 676.
- 64Jiao X, Nawab O, Patel T, et al. Recent advances targeting CCR5 for cancer and its role in Immuno-oncology. Cancer Res. 2019; 79(19): 4801-4807.
- 65You Y, Li Y, Li M, et al. Ovarian cancer stem cells promote tumour immune privilege and invasion via CCL5 and regulatory T cells. Clin Exp Immunol. 2018; 191(1): 60-73.
- 66Solis-Castillo LA, Garcia-Romo GS, Diaz-Rodriguez A, et al. Tumor-infiltrating regulatory T cells, CD8/Treg ratio, and cancer stem cells are correlated with lymph node metastasis in patients with early breast cancer. Breast Cancer. 2020; 27(5): 837-849.
- 67Foulds GA, Vadakekolathu J, Abdel-Fatah TMA, et al. Immune-phenotyping and transcriptomic profiling of peripheral blood mononuclear cells from patients with breast cancer: identification of a 3 gene signature which predicts relapse of triple negative breast cancer. Front Immunol. 2018; 9: 2028.
- 68Tang Y, Xu Q, Hu L, et al. Tumor microenvironment-derived R-spondins enhance antitumor immunity to suppress tumor growth and sensitize for immune checkpoint blockade therapy. Cancer Discov. 2021; 11(12): 3142-3157.
- 69Shokouhifar A, Firouzi J, Nouri M, Sarab GA, Ebrahimi M. NK cell upraise in the dark world of cancer stem cells. Cancer Cell Int. 2021; 21(1): 682.
- 70Secchiari F, Nuñez SY, Sierra JM, et al. The MICA-NKG2D axis in clear cell renal cell carcinoma bolsters MICA as target in immuno-oncology. Onco Targets Ther. 2022; 11(1):2104991.
- 71Luo Q, Luo W, Zhu Q, et al. Tumor-derived soluble MICA obstructs the NKG2D pathway to restrain NK cytotoxicity. Aging Dis. 2020; 11(1): 118-128.
- 72Hwang WL, Lan HY, Cheng WC, Huang SC, Yang MH. Tumor stem-like cell-derived exosomal RNAs prime neutrophils for facilitating tumorigenesis of colon cancer. J Hematol Oncol. 2019; 12(1): 10.
- 73Ren Z, Chen Y, Shi L, et al. Sox9/CXCL5 axis facilitates tumour cell growth and invasion in hepatocellular carcinoma. FEBS J. 2022; 289(12): 3535-3549.
- 74Shimizu M, Tanaka N. IL-8-induced O-GlcNAc modification via GLUT3 and GFAT regulates cancer stem cell-like properties in colon and lung cancer cells. Oncogene. 2019; 38(9): 1520-1533.
- 75Yang Y, Li X, Wang T, Guo Q, Xi T, Zheng L. Emerging agents that target signaling pathways in cancer stem cells. J Hematol Oncol. 2020; 13(1): 60.
- 76Jiang N, Zou C, Zhu Y, et al. HIF-1ɑ-regulated miR-1275 maintains stem cell-like phenotypes and promotes the progression of LUAD by simultaneously activating Wnt/β-catenin and Notch signaling. Theranostics. 2020; 10(6): 2553-2570.
- 77Adebayo Michael AO, Ko S, Tao J, et al. Inhibiting glutamine-dependent mTORC1 activation ameliorates liver cancers driven by β-catenin mutations. Cell Metab. 2019; 29(5): 1135-1150.
- 78Lv J, Liu Y, Cheng F, et al. Cell softness regulates tumorigenicity and stemness of cancer cells. EMBO J. 2021; 40(2):e106123.
- 79Zhuang X, Zhang H, Li X, et al. Differential effects on lung and bone metastasis of breast cancer by Wnt signalling inhibitor DKK1. Nat Cell Biol. 2017; 19(10): 1274-1285.
- 80Perry JM, Tao F, Roy A, et al. Overcoming Wnt-β-catenin dependent anticancer therapy resistance in leukaemia stem cells. Nat Cell Biol. 2020; 22(6): 689-700.
- 81Doan P, Musa A, Murugesan A, et al. Glioblastoma multiforme stem cell cycle arrest by alkylaminophenol through the modulation of EGFR and CSC signaling pathways. Cell. 2020; 9(3): 681.
- 82Arend RC, Londoño-Joshi AI, Gangrade A, et al. Niclosamide and its analogs are potent inhibitors of Wnt/β-catenin, mTOR and STAT3 signaling in ovarian cancer. Oncotarget. 2016; 7(52): 86803-86815.
- 83Chen L, Chan LS, Lung HL, et al. Crucifera sulforaphane (SFN) inhibits the growth of nasopharyngeal carcinoma through DNA methyltransferase 1 (DNMT1)/Wnt inhibitory factor 1 (WIF1) axis. Phytomedicine. 2019; 63:153058.
- 84Tuynman JB, Vermeulen L, Boon EM, et al. Cyclooxygenase-2 inhibition inhibits c-met kinase activity and Wnt activity in colon cancer. Cancer Res. 2008; 68(4): 1213-1220.
- 85Drew DA, Schuck MM, Magicheva-Gupta MV, et al. Effect of low-dose and standard-dose aspirin on PGE biosynthesis among individuals with colorectal adenomas: a randomized clinical trial. Cancer Prev Res (Phila). 2020; 13(10): 877-888.
- 86Smith ML, Hawcroft G, Hull MA. The effect of non-steroidal anti-inflammatory drugs on human colorectal cancer cells: evidence of different mechanisms of action. Eur J Cancer. 2000; 36(5): 664-674.
- 87Balaguer F, Stoffel EM, Burke CA, et al. Combination of sulindac and eflornithine delays the need for lower gastrointestinal surgery in patients with familial adenomatous polyposis: post hoc analysis of a randomized clinical trial. Dis Colon Rectum. 2022; 65(4): 536-545.
- 88Burke CA, Dekker E, Lynch P, et al. Eflornithine plus sulindac for prevention of progression in familial adenomatous polyposis. N Engl J Med. 2020; 383(11): 1028-1039.
- 89Rodon J, Argilés G, Connolly RM, et al. Phase 1 study of single-agent WNT974, a first-in-class porcupine inhibitor, in patients with advanced solid tumours. Br J Cancer. 2021; 125(1): 28-37.
- 90Clara JA, Monge C, Yang Y, Takebe N. Targeting signalling pathways and the immune microenvironment of cancer stem cells - a clinical update. Nat Rev Clin Oncol. 2020; 17(4): 204-232.
- 91Fendler A, Bauer D, Busch J, et al. Inhibiting WNT and NOTCH in renal cancer stem cells and the implications for human patients. Nat Commun. 2020; 11(1): 929.
- 92Ibrahim SA, Gadalla R, El-Ghonaimy EA, et al. Syndecan-1 is a novel molecular marker for triple negative inflammatory breast cancer and modulates the cancer stem cell phenotype via the IL-6/STAT3, Notch and EGFR signaling pathways. Mol Cancer. 2017; 16(1): 57.
- 93Takebe N, Miele L, Harris PJ, et al. Targeting Notch, Hedgehog, and Wnt pathways in cancer stem cells: clinical update. Nat Rev Clin Oncol. 2015; 12(8): 445-464.
- 94Liu J, Mao Z, Huang J, Xie S, Liu T, Mao Z. Blocking the NOTCH pathway can inhibit the growth of CD133-positive A549 cells and sensitize to chemotherapy. Biochem Biophys Res Commun. 2014; 444(4): 670-675.
- 95Aoki S, Mizuma M, Takahashi Y, et al. Aberrant activation of Notch signaling in extrahepatic cholangiocarcinoma: clinicopathological features and therapeutic potential for cancer stem cell-like properties. BMC Cancer. 2016; 16(1): 854.
- 96Barat S, Chen X, Cuong Bui K, et al. Gamma-secretase inhibitor IX (GSI) impairs concomitant activation of Notch and Wnt-Beta-catenin pathways in CD44 gastric cancer stem cells. Stem Cells Transl Med. 2017; 6(3): 819-829.
- 97Kummar S, O'Sullivan Coyne G, Do KT, et al. Clinical activity of the γ-secretase inhibitor PF-03084014 in adults with desmoid tumors (aggressive fibromatosis). J Clin Oncol. 2017; 35(14): 1561-1569.
- 98Lee SM, Moon J, Redman BG, et al. Phase 2 study of RO4929097, a gamma-secretase inhibitor, in metastatic melanoma: SWOG 0933. Cancer. 2015; 121(3): 432-440.
- 99Biktasova AK, Dudimah DF, Uzhachenko RV, et al. Multivalent forms of the Notch ligand DLL-1 enhance antitumor T-cell immunity in lung cancer and improve efficacy of EGFR-targeted therapy. Cancer Res. 2015; 75(22): 4728-4741.
- 100Kangsamaksin T, Murtomaki A, Kofler NM, et al. NOTCH decoys that selectively block DLL/NOTCH or JAG/NOTCH disrupt angiogenesis by unique mechanisms to inhibit tumor growth. Cancer Discov. 2015; 5(2): 182-197.
- 101Chiorean EG, LoRusso P, Strother RM, et al. A phase I first-in-human study of enoticumab (REGN421), a fully human delta-like ligand 4 (Dll4) monoclonal antibody in patients with advanced solid tumors. Clin Cancer Res. 2015; 21(12): 2695-2703.
- 102Morgensztern D, Besse B, Greillier L, et al. Efficacy and safety of rovalpituzumab tesirine in third-line and beyond patients with DLL3-expressing, relapsed/refractory small-cell lung cancer: results from the phase II TRINITY study. Clin Cancer Res. 2019; 25(23): 6958-6966.
- 103Lee SH, Nam HJ, Kang HJ, Kwon HW, Lim YC. Epigallocatechin-3-gallate attenuates head and neck cancer stem cell traits through suppression of Notch pathway. Eur J Cancer. 2013; 49(15): 3210-3218.
- 104Sun T, Patil R, Galstyan A, et al. Blockade of a Laminin-411-Notch axis with CRISPR/Cas9 or a nanobioconjugate inhibits glioblastoma growth through tumor-microenvironment cross-talk. Cancer Res. 2019; 79(6): 1239-1251.
- 105Wang H, Yang M, Lin L, et al. HepG2 cells acquire stem cell-like characteristics after immune cell stimulation. Cell Oncol (Dordr). 2016; 39(1): 35-45.
- 106Lu Y, Zhu Y, Deng S, et al. Targeting the sonic hedgehog pathway to suppress the expression of the cancer stem cell (CSC)-related transcription factors and CSC-driven thyroid tumor growth. Cancer. 2021; 13(3): 418.
- 107Almazán-Moga A, Zarzosa P, Vidal I, et al. Hedgehog pathway inhibition hampers sphere and holoclone formation in rhabdomyosarcoma. Stem Cells Int. 2017; 2017:7507380.
- 108Sekulic A, Migden MR, Basset-Seguin N, et al. Long-term safety and efficacy of vismodegib in patients with advanced basal cell carcinoma: final update of the pivotal ERIVANCE BCC study. BMC Cancer. 2017; 17(1): 332.
- 109Sekulic A, Migden MR, Lewis K, et al. Pivotal ERIVANCE basal cell carcinoma (BCC) study: 12-month update of efficacy and safety of vismodegib in advanced BCC. J Am Acad Dermatol. 2015; 72(6): 1021-1026.
- 110Ben Ishai M, Tiosano A, Fenig E, Ben Simon G, Yassur I. Outcomes of Vismodegib for periocular locally advanced basal cell carcinoma from an open-label trial. JAMA Ophthalmol. 2020; 138(7): 749-755.
- 111Marescassier H, Dousset L, Beylot-Barry M, et al. Predictive factors of non-response to Vismodegib in locally advanced basal-cell carcinoma. Dermatology. 2021; 237(6): 1023-1028.
- 112Frappaz D, Barritault M, Montané L, et al. MEVITEM-a phase I/II trial of vismodegib + temozolomide vs temozolomide in patients with recurrent/refractory medulloblastoma with sonic hedgehog pathway activation. Neuro Oncol. 2021; 23(11): 1949-1960.
- 113Gutzmer R, Loquai C, Robert C, et al. Key clinical adverse events in patients with advanced basal cell carcinoma treated with Sonidegib or Vismodegib: a post hoc analysis. Dermatol Ther (Heidelb). 2021; 11(5): 1839-1849.
- 114Danial C, Sarin KY, Oro AE, Chang ALS. An investigator-initiated open-label trial of Sonidegib in advanced basal cell carcinoma patients resistant to Vismodegib. Clin Cancer Res. 2016; 22(6): 1325-1329.
- 115Han J, Won M, Kim JH, et al. Cancer stem cell-targeted bio-imaging and chemotherapeutic perspective. Chem Soc Rev. 2020; 49(22): 7856-7878.
- 116Lenos KJ, Miedema DM, Lodestijn SC, et al. Stem cell functionality is microenvironmentally defined during tumour expansion and therapy response in colon cancer. Nat Cell Biol. 2018; 20(10): 1193-1202.
- 117Hanahan D. Hallmarks of cancer: new dimensions. Cancer Discov. 2022; 12(1): 31-46.
- 118Douglas H, Weinberg Robert A. Hallmarks of cancer: the next generation. Cell. 2011; 144(5): 646-674.
- 119Warburg O. On respiratory impairment in cancer cells. Science. 1956; 124(3215): 269-270.
- 120Jones CL, Inguva A, Jordan CT. Targeting energy metabolism in cancer stem cells: progress and challenges in leukemia and solid tumors. Cell Stem Cell. 2021; 28(3): 378-393.
- 121Brown JR, Chan DK, Shank JJ, et al. Phase II clinical trial of metformin as a cancer stem cell-targeting agent in ovarian cancer. JCI Insight. 2020; 5(11):e133247.
- 122Man SM, Jenkins BJ. Context-dependent functions of pattern recognition receptors in cancer. Nat Rev Cancer. 2022; 22(7): 397-413.
- 123Bhatt AP, Redinbo MR, Bultman SJ. The role of the microbiome in cancer development and therapy. CA Cancer J Clin. 2017; 67(4): 326-344.
- 124Wang H, Rong X, Zhao G, et al. The microbial metabolite trimethylamine N-oxide promotes antitumor immunity in triple-negative breast cancer. Cell Metab. 2022; 34(4): 581-594.
- 125Hezaveh K, Shinde RS, Klötgen A, et al. Tryptophan-derived microbial metabolites activate the aryl hydrocarbon receptor in tumor-associated macrophages to suppress anti-tumor immunity. Immunity. 2022; 55(2): 324-340.
- 126Harrington K, Freeman DJ, Kelly B, Harper J, Soria JC. Optimizing oncolytic virotherapy in cancer treatment. Nat Rev Drug Discov. 2019; 18(9): 689-706.
- 127Liu YT, Sun ZJ. Turning cold tumors into hot tumors by improving T-cell infiltration. Theranostics. 2021; 11(11): 5365-5386.
- 128Meylan M, Petitprez F, Becht E, et al. Tertiary lymphoid structures generate and propagate anti-tumor antibody-producing plasma cells in renal cell cancer. Immunity. 2022; 55(3): 527-541.
- 129Calderaro J, Petitprez F, Becht E, et al. Intra-tumoral tertiary lymphoid structures are associated with a low risk of early recurrence of hepatocellular carcinoma. J Hepatol. 2019; 70(1): 58-65.
- 130Ding GY, Ma JQ, Yun JP, et al. Distribution and density of tertiary lymphoid structures predict clinical outcome in intrahepatic cholangiocarcinoma. J Hepatol. 2022; 76(3): 608-618.