Dual Photoredox/Copper-Catalyzed 1,2-Diphosphorothiolation of Alkenes with P(O)SH Compounds to Access Vicinal Bisphosphorothioates
Corresponding Author
Pengbo Zhang
School of Public Health, Xinxiang Medical University, Xinxiang, Henan, 453003 China
E-mail: [email protected]; [email protected]Search for more papers by this authorLongyu Wang
School of Public Health, Xinxiang Medical University, Xinxiang, Henan, 453003 China
Search for more papers by this authorXinyi Guo
School of Public Health, Xinxiang Medical University, Xinxiang, Henan, 453003 China
Search for more papers by this authorYaxin Liu
School of Public Health, Xinxiang Medical University, Xinxiang, Henan, 453003 China
Search for more papers by this authorQihang Yang
School of Public Health, Xinxiang Medical University, Xinxiang, Henan, 453003 China
Search for more papers by this authorCorresponding Author
Xia Gao
School of Public Health, Xinxiang Medical University, Xinxiang, Henan, 453003 China
E-mail: [email protected]; [email protected]Search for more papers by this authorCorresponding Author
Pengbo Zhang
School of Public Health, Xinxiang Medical University, Xinxiang, Henan, 453003 China
E-mail: [email protected]; [email protected]Search for more papers by this authorLongyu Wang
School of Public Health, Xinxiang Medical University, Xinxiang, Henan, 453003 China
Search for more papers by this authorXinyi Guo
School of Public Health, Xinxiang Medical University, Xinxiang, Henan, 453003 China
Search for more papers by this authorYaxin Liu
School of Public Health, Xinxiang Medical University, Xinxiang, Henan, 453003 China
Search for more papers by this authorQihang Yang
School of Public Health, Xinxiang Medical University, Xinxiang, Henan, 453003 China
Search for more papers by this authorCorresponding Author
Xia Gao
School of Public Health, Xinxiang Medical University, Xinxiang, Henan, 453003 China
E-mail: [email protected]; [email protected]Search for more papers by this authorComprehensive Summary
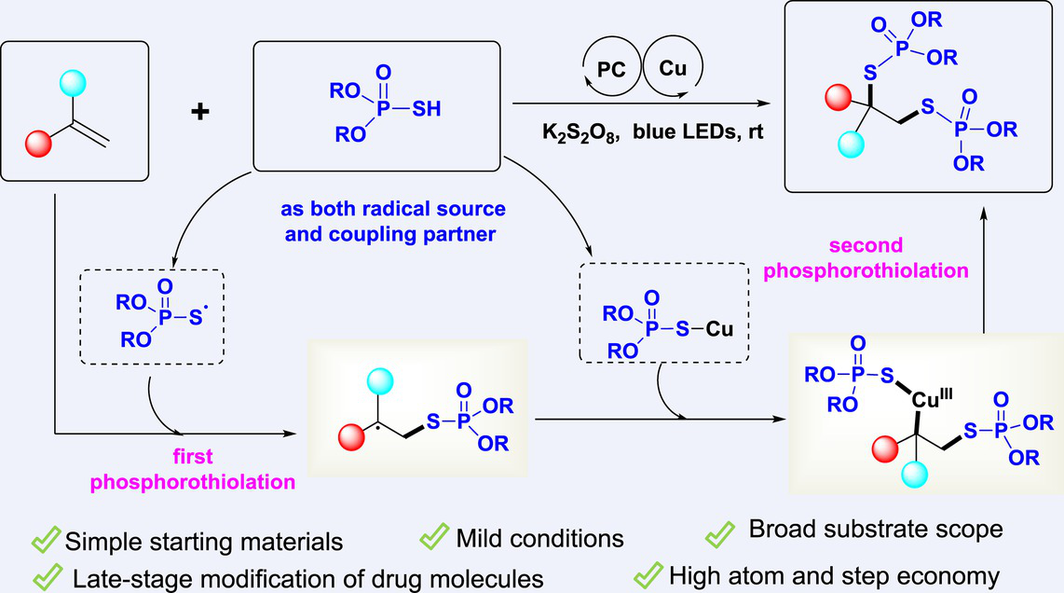
An efficient photoredox/copper dual-catalyzed 1,2-diphosphorothiolation of alkenes with P(O)SH compounds was realized under oxidative conditions. In this transformation, P(O)SH acted as both the phosphorothioate radical source and the coupling partner. A wide range of valuable vicinal bisphosphorothioates can be easily constructed starting from simple raw materials in a step- and atom-economical manner. Notably, this reaction system has been successfully used to incorporate two phosphorothioate groups into many drug molecules, highlighting the substantial synthetic potential of this protocol.
Supporting Information
| Filename | Description |
|---|---|
| cjoc202500271-sup-0001-supinfo.pdfPDF document, 6 MB |
Appendix S1: Supporting Information |
Please note: The publisher is not responsible for the content or functionality of any supporting information supplied by the authors. Any queries (other than missing content) should be directed to the corresponding author for the article.
References
- 1(a) Li, N.-S.; Frederiksen, J. K.; Piccirilli, J. A. Synthesis, Properties, and Applications of Oligonucleotides Containing an RNA Dinucleotide Phosphorothiolate Linkage. Acc. Chem. Res. 2011, 44, 1257–1269; (b) Shi, S.; Chen, J.; Zhuo, S.; Wu, Z.; Fang, M.; Tang, G.; Zhao, Y. Lodidecatalyzed Phosphorothiolation of Heteroarenes Using P(O)H Compounds and Elemental Sulfur. Adv. Synth. Catal. 2019, 361, 3210–3216; (c) Kumar, T. S.; Yang, T.; Mishra, S.; Cronin, C.; Chakraborty, S.; Shen, J.-B.; Liang, B. T.; Jacobson, K. A. 5′-Phosphate and 5′-Phosphonate Ester Derivatives of (N)-Methanocarba Adenosine with in Vivo Cardioprotective Activity. J. Med. Chem. 2013, 56, 902–914; (d) Lauer, A. M.; Mahmud, F.; Wu, J. Cu (I)-Catalyzed, α-Selective, Allylic Alkylation Reactions between Phosphorothioate Esters and Organomagnesium Reagents. J. Am. Chem. Soc. 2011, 133, 9119–9123; (e) Lauer, A. M.; Wu, J. Palladium-Catalyzed Allylic Fluorination of Cinnamyl Phosphorothioate Esters. Org. Lett. 2012, 14, 5138–5141.
- 2(a) Quin, L. D. A Guide to Organophosphorus Chemistry, Wiley, 2000; (b) Kaboudin, B.; Emadi, S.; Hadizadeh, A. Synthesis of Novel Phosphorothioates and Phosphorodithioates and Their Differential Inhibition of Cholinesterases. Bioorg. Chem. 2009, 37, 101–105; (c) Murdock, L.; Hopkins, T. Insecticidal, Anticholinesterase, and Hydrolytic Properties of O,O-Dialkyl S-Aryl Phosphorothiolates in Relation to Structure. J. Agric. Food Chem. 1968, 16, 954–958; (d) Huangfu, X.; Zhang, Y.; Chen, P.; Lu, G.; Cao, Y.; Tang, G.; Zhao, Y. Synthesis of Mixed Phosphorotrithioates from White Phosphorus. Green Chem. 2020, 22, 8353–8359.
- 3(a) dos Santos, V. M.; Sant’Anna, C. M. R.; Borja, G. E. M.; Chaaban, A.; Côrtes, W. S.; DaCosta, J. B. N. New Bisphosphorothioates and Bisphosphoroamidates: Synthesis, Molecular Modeling and Determination of Insecticide and Toxicological Profile. Bioorg. Chem. 2007, 35, 68–81; (b) Zhao, Q.; Xie, R.; Zhang, T.; Fang, J.; Mei, X.; Ning, J.; Tang, Y. Homo-and Hetero-Dimers of Inactive Organophosphorous Group Binding at Dual Sites of AChE. Bioorg. Med. Chem. Lett. 2011, 21, 6404–6408; (c) Xie, R.; Zhao, Q.; Zhang, T.; Fang, J.; Mei, X.; Ning, J.; Tang, Y., Design, Synthesis and Biological Evaluation of Organophosphorous-Homodimers as Dual Binding Site Acetylcholinesterase Inhibitors. Bioorg. Med. Chem. 2013, 21, 278–282.
- 4(a) Xu, J.; Zhang, L.; Li, X.; Gao, Y.; Tang, G.; Zhao, Y. Phosphorothiolation of Aryl Boronic Acids Using P (O) H Compounds and Elemental Sulfur. Org. Lett. 2016, 18, 1266–1269; (b) Zhu, Y.; Chen, T.; Li, S.; Shimada, S.; Han, L.-B. Efficient Pd-Catalyzed Dehydrogenative Coupling of P(O)H with RSH: A Precise Construction of P(O)–S Bonds. J. Am. Chem. Soc. 2016, 138, 5825–5828; (c) Song, S.; Zhang, Y.; Yeerlan, A.; Zhu, B.; Liu, J.; Jiao, N. Cs2CO3-Catalyzed Aerobic Oxidative Cross-DeHydrogenative Coupling of Thiols with Phosphonates and Arenes. Angew. Chem. 2017, 129, 2527–2531; (d) Jones, D. J.; O'Leary, E. M.; O'Sullivan, T. P. Synthesis and Application of Phosphonothioates, Phosphonodithioates, Phosphorothioates, Phosphinothioates and Related Compounds. Tetrahedron Lett. 2018, 59, 4279–4292; (e) Jones, D. J.; O'Leary, E. M.; O'Sullivan, T. P. Modern Synthetic Approaches to Phosphorus-sulfur Bond Formation in Organophosphorus Compounds. Adv. Synth. Catal. 2020, 362, 2801–2846; (f) Yue, H.-Q.; Shi, D.-W.; Zhang, P.; Xiao, B.; Jia, L.-T.; Li, R.; Zhao, S.-N.; Yang, S.-D.; Yang, B., DMSO-Catalyzed Double P–O Bond or Double P–S Bond Formations of Phosphinic Acids. Org. Lett. 2024, 26, 8939–8944; (g) Zhu, L.; Luo, W.; Guo, F.; Chen, L.; Tang, Y.; Xiong, B.; Liu, Y.; Tang, K.-W.; Qiu, R. Halide-Free and Metal-Free Allylic Thiolation/Selenation of P (O) H Compounds with Sulfur/Selenium and Allylic Alcohols. Green Chem. 2024, 26, 10886–10892; (h) Xiong, B.; Zheng, S.; Xu, W.; Liu, Y.; Zhu, L.; Tang, K.-W.; Sun, Z.; Wong, W.-Y. Copper-Catalyzed Phosphorothiolation/Seleno (Telluro) Phosphorylation of Vinylsulfonium Salts with P(III)-Nucleophiles via the Insertion of Elemental Sulfur/Selenium/Tellurium. Org. Chem. Front. 2025, 12, 936–946; (i) Guo, Y.; Luo, Y.; Mu, S.; Su, J.; Xu, J.; Song, Q. Design, Synthesis, and Applications of Ortho-Sulfur Substituted Arylphosphanes. CCS Chem. 2023, 5, 1353–1364.; (j) Guo, Y.; Lin, G.; Zhang, M.; Xu, J.; Song, Q. Photo-Induced DecarboxyLative CS Bond Formation to Access Sterically Hindered Unsymmetric S-Alkyl Thiosulfonates and SS-Alkyl Thiosulfonates. Nat. Commun. 2024, 15, 7313.
- 5(a) Volkov, P.; Ivanova, N.; Gusarova, N.; Trofimov, B. A Simple Route to Dithiophosphinic Esters and Diesters from Secondary Phosphine Sulfides and Thiols or Dithiols. J. Sulfur Chem. 2014, 35, 237−247; (b) Qiu, Y.; Worch, J. C.; Chirdon, D. N.; Kaur, A.; Maurer, A. B.; Amsterdam, S.; Collins, C. R.; Pintauer, T.; Yaron, D.; Bernhard, S.; Noonan, K. J. T. Tuning Thiophene with Phosphorus: Synthesis and Electronic Properties of Benzobisthiaphospholes. Chem.-Eur. J. 2014, 20, 7746−7751.
- 6(a) Liu, X.; Zhou, L.; Yang, R.; Song, X. R.; Xiao, Q. Recent Advances in the Direct Synthesis of Sulfur-Containing Organophosphorus Compounds via Radical Processes. Adv. Synth. Catal. 2023, 365, 2280–2298; (b) Zheng, H.; Peng, J.; Liu, X.; Zhao, P. Recent Advances on Direct Phosphorothiolation Reactions. Adv. Synth. Catal. 2024, 366, 1–23; (c) Sarkar, B.; Hajra, A. Hydro-Phosphorothiolation of Styrene and Cyclopropane with S-Hydrogen Phosphorothioates under Ambient Conditions. Org. Lett. 2024, 26, 5141–5145; (d) Zhang, Y.; Du, S.; Yang, T.; Jin, F.; Zhou, J.; Cao, B.; Mao, Z.-J.; Song, X.-R.; Xiao, Q. Direct and Efficient Synthesis of Tetrasubstituted Allenyl Organothiophosphates from Propargylic Alcohols under Catalyst- and Additive- Free Conditions. Org. Chem. Front. 2022, 9, 3156–3162; (e) Zheng, Z.; He, J.; Ma, Q.; Zhang, Y.; Liu, Y.; Tang, G.; Zhao, Y. Photoredox/Copper-Catalyzed Coupling of Terminal Alkynes with P(O)SH Compounds Leading to Alkynyl Phosphorothioates. Green Chem. 2022, 24, 4484–4489; (f) Du, S.; Jiang, S.; Yang, R.; Jin, F.; Huang, H.; Tian, W.-F.; Zhou, Z.-Z.; Song, X.-R.; Xiao, Q. Direct Synthesis of Benzo[b]Fluorenyl Thiophosphates via Tandem Cyclization of Diynols with (RO)2P(O)SH. Org. Lett. 2023, 25, 1263–1267.
- 7(a) Guo, Y.; Luo, Y.; Mu, S.; Xu, J.; Song, Q. Photoinduced Decarboxylative Phosphorothiolation of N-Hydroxyphthalimide Esters. Org. Lett. 2021, 23, 6729–6734; (b) Zheng, Z.; Shi, S; Ma, Q.; Yang, Y.; Liu, Y.; Tang, G.; Zhao, Y. Synthesis of δ-Phosphorothiolated Alcohols by Photoredox/Copper Catalyzed Remote C(sp3)–H Phosphorothiolation of N-Alkoxypyridinium Salts. Org. Chem. Front. 2021, 8, 6845–6850; (c) Piedra, H. F.; Gebler, V.; Valdés, C.; Plaza, M. Photochemical Halogen-Bonding Assisted Carbothiophosphorylation Reactions of Alkenyl and 1,3-Dienyl Bromides. Chem. Sci. 2023, 14, 12767–12773; (d) Shi, S.; Chen, H.; Zhao, M.; Yang, S.; Li, P.; Wang, X.; Zhu, J.; Fang, Q.; Xu, W.; Tang, G.; Gao, Y. Copper-Catalyzed Fluoroalkylphosphorothiolation of Alkynes for the Synthesis of (E)-β-Fluoroalkyl Vinyl Phosphorothioates. Org. Lett. 2023, 25, 8296–8301; (e) Shi, S.; Chen, H.; Yang, S.; Dong, H.; Zhu, J.; Zheng, B.; Wang, X.; Liang, Z.; Ren, H.; Gao, Y. Photoredox/Copper Dual-Catalyzed Phosphorothiolation of Propargylic Derivatives for the Switchable Synthesis of S-Alkyl, S-Vinyl and S-Allenyl Phosphorothioates. Org. Lett. 2024, 26, 7049–7054; (f) Guo, G.; Ma, J.; Dong, Y.; Wu, Q.; Lv, J.; Shi, Y.; Yang, D. Visible Light/Copper Catalysis-Enabled Arylation and Alkenylation of Phosphorothioates via Site-SeLective C–H Thianthrenation. Org. Lett. 2024, 26, 8382–8388; (g) Kumar Ghosh, A.; Neogi, S.; Ghosh, P.; Hajra, A. Synergistic Photoredox and Iron (II) Catalyzed Carbo-Phosphorothiolation of Vinyl Arenes. Adv. Synth. Catal. 2023, 365, 2271–2278; (h) Peng, S.; Yang, L. H.; Xu, X. Q.; Xie, L. Y. Multi-Component Heteroarylphosphorothiolation of Alkenes for Accessing β-Phosphorothiolated Quinoxalinones. Adv. Synth. Catal. 2024, 366, 2758–2763; (i) Mo, X.; Guo, R.; Zhang, G. Recent Developments in Copper(I)-Catalyzed Enantioselective Alkynylation Reactions via a Radical Process. Chin. J. Chem. 2023, 41, 481–489; (j) Huang, C.; Wan, Z.; Zhu, A.; Chen, C. Copper Catalyzed Enantioconvergent Nucleophilic Substitutions. Chin. J. Chem. 2024, 42, 1161–1174.
- 8(a) Li, Z.-L.; Fang, G.-C.; Gu, Q.-S.; Liu, X.-Y. Recent Advances in Copper-Catalysed Radical-Involved Asymmetric 1,2-Difunctionalization of Alkenes. Chem. Soc. Rev. 2020, 49, 32–48; (b) Gupta, S.; Kundu, A.; Ghosh, S.; Chakraborty, A.; Hajra, A. Visible Light-Induced Organophotoredox-Catalyzed Difunctionalization of Alkenes and Alkynes. Green Chem. 2023, 25, 8459–8493.
- 9 Ju, T.; Zhou, Y.-Q.; Cao, K.-G.; Fu, Q.; Ye, J.-H.; Sun, G.-Q.; Liu, X.-F.; Chen, L.; Liao, L.-L.; Yu, D.-G. Dicarboxylation of Alkenes, Allenes and (Hetero)Arenes with CO2 via Visible-Light Photoredox Catalysis. Nat. Catal. 2021, 4, 304–311.
- 10
Lian, P.; Long, W.; Li, J.; Zheng, Y.; Wan, X. Visible-Light-Induced Vicinal Dichlorination of Alkenes through LMCT Excitation of CuCl2. Angew. Chem. Int. Ed. 2020, 132, 23809–23814.
10.1002/ange.202010801 Google Scholar
- 11 Zhang, M.; Zhang, J.; Li, Q.; Shi, Y. Iron-Mediated Ligand-to-Metal Charge Transfer Enables 1,2-Diazidation of Alkenes. Nat. Commun. 2022, 13, 7880.
- 12 Chen, F.; Jiang, L.; Hu, C.; Liu, J.; Yi, W. Photocatalyzed Ditrifluoromethylthiolation of Alkenes with CF3SO2Na. Sci. China Chem. 2023, 67, 587–594.
- 13(a) Zhang, P.; Yu, G.; Li, W.; Shu, Z.; Wang, L.; Li, Z.; Gao, X. Copper-Catalyzed Multicomponent Trifluoromethylphosphorothiolation of Alkenes: Access to CF3-Containing Alkyl Phosphorothioates. Org. Lett. 2021, 23, 5848–5852; (b) Zhang, P.; Li, W.; Qu, W.; Shu, Z.; Tao, Y.; Lin, J.; Gao, X. Copper and Photocatalytic Radical Relay Enabling Fluoroal-kylphosphorothiolation of Alkenes: Modular Synthesis of Fluorine-Containing S-Alkyl Phosphorothioates and Phosphorodithioates. Org. Lett. 2021, 23, 9267–9272.
- 14 Zhang, P.; Qu, W.; Yang, S.; Wang, L.; Zhang, L.; Zhu, X.; Gao, X. Additive-Free, N-Chlorosuccinimide-Promoted Electrophilic Phosphorothiolation/Cyclization of Alkynes with P(O)SH Compounds to Access Heterocyclic Phosphorothiolated Molecules. Org. Chem. Front. 2024, 11, 5841–5846.




