Visible-Light-Driven Multicomponent Reactions of Diazosulfonium Triflates with Amines and CS2 or CO2: Direct Synthesis of Bis-Dithiocarbamates/Carbamates
Xue-Cen Xu
Jilin Province Key Laboratory of Organic Functional Molecular Design & Synthesis, Faculty of Chemistry, Northeast Normal University, Changchun, Jilin, 130024 China
Search for more papers by this authorYue-Gong
Jilin Province Key Laboratory of Organic Functional Molecular Design & Synthesis, Faculty of Chemistry, Northeast Normal University, Changchun, Jilin, 130024 China
Search for more papers by this authorJie Wang
Jilin Province Key Laboratory of Organic Functional Molecular Design & Synthesis, Faculty of Chemistry, Northeast Normal University, Changchun, Jilin, 130024 China
Search for more papers by this authorYu-Xuan Meng
Jilin Province Key Laboratory of Organic Functional Molecular Design & Synthesis, Faculty of Chemistry, Northeast Normal University, Changchun, Jilin, 130024 China
Search for more papers by this authorCorresponding Author
Yu-Long Zhao
Jilin Province Key Laboratory of Organic Functional Molecular Design & Synthesis, Faculty of Chemistry, Northeast Normal University, Changchun, Jilin, 130024 China
E-mail: [email protected]Search for more papers by this authorXue-Cen Xu
Jilin Province Key Laboratory of Organic Functional Molecular Design & Synthesis, Faculty of Chemistry, Northeast Normal University, Changchun, Jilin, 130024 China
Search for more papers by this authorYue-Gong
Jilin Province Key Laboratory of Organic Functional Molecular Design & Synthesis, Faculty of Chemistry, Northeast Normal University, Changchun, Jilin, 130024 China
Search for more papers by this authorJie Wang
Jilin Province Key Laboratory of Organic Functional Molecular Design & Synthesis, Faculty of Chemistry, Northeast Normal University, Changchun, Jilin, 130024 China
Search for more papers by this authorYu-Xuan Meng
Jilin Province Key Laboratory of Organic Functional Molecular Design & Synthesis, Faculty of Chemistry, Northeast Normal University, Changchun, Jilin, 130024 China
Search for more papers by this authorCorresponding Author
Yu-Long Zhao
Jilin Province Key Laboratory of Organic Functional Molecular Design & Synthesis, Faculty of Chemistry, Northeast Normal University, Changchun, Jilin, 130024 China
E-mail: [email protected]Search for more papers by this authorComprehensive Summary
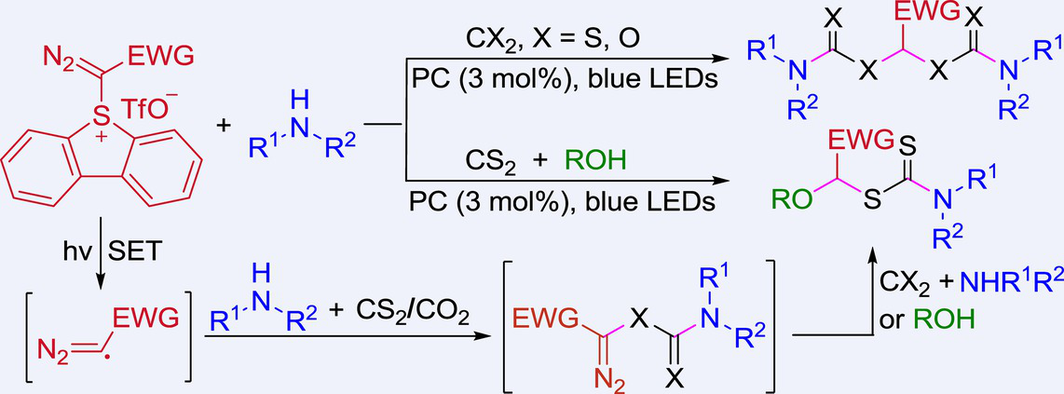
Visible light-induced transformation of CO2 and CS2 into value-added products has attracted worldwide attention because it mimics nature. In this context, although visible-light-induced direct synthesis of dithiocarbamates and carbamates employing SO2 and CO2 as a C1 source has been reported, all these reactions are limited to the preparation of S-alkyl mono-dithiocarbamates and O-alkyl mono-carbamates. Herein, we report a visible-light photoredox-catalyzed multicomponent reaction of diazosulfonium triflates with amines and CS2 or CO2. Mechanistic studies indicate that the diazomethyl radicals might be generated as the key intermediates, thus providing a direction for the application of diazomethyl radicals with other radical acceptors.
Supporting Information
| Filename | Description |
|---|---|
| cjoc202500235-sup-0001-supinfo.pdfPDF document, 9.5 MB |
Appendix S1: Supporting Information |
Please note: The publisher is not responsible for the content or functionality of any supporting information supplied by the authors. Any queries (other than missing content) should be directed to the corresponding author for the article.
References
- 1(a) Lincke, T.; Behnken, S.; Ishida, K.; Roth, M.; Hertweck, C. Closthioamide: An Unprecedented Polythioamide Antibiotic from the Strictly Anaerobic Bacterium Clostridium cellulolyticum. Angew. Chem. Int. Ed. 2010, 49, 2011–2013; (b) Bach, A.; Eildal, J. N. N.; Stuhr-Hansen, N.; Deeskamp, R.; Gottschalk, M.; Pedersen, S. W.; Kristensen, A. S.; Strømgaard, K. Cell-Permeable and Plasma-Stable Peptidomimetic Inhibitors of the Postsynaptic Density-95/N-Methyl-D-Aspartate Receptor Interaction. J. Med. Chem. 2011, 54, 1333–1346; (c) Tran, C.; Ouk, S.; Clegg, N. J.; Chen, Y.; Watson, P. A.; Arora, V.; Wongvipat, J.; Smith-Jones, P. M.; Yoo, D.; Kwon, A.; Wasielewska, T.; Welsbie, D.; Chen, C. D.; Higano, C. S.; Beer, T. M. D.; Hung, T.; Scher, H. I.; Jung, M. E.; Sawyers, C. L. Development of a Second- Generation Antiandrogen for Treatment of Advanced Prostate Cancer. Science 2009, 324, 787–790; (d) Chi, Y. H.; Lee, H.; Paik, S. H.; Lee, J. H.; Yoo, B. W.; Kim, J. H.; Tan, H. K.; Kim, S. L. Safety, Tolerability, Pharmacokinetics, and Pharmacodynamics of Fimasartan Following Single and Repeated Oral Administration in the Fasted and Fed States in Healthy Subjects. Am. J. Cardiovasc. Drugs 2011, 11, 335–346.
- 2For selected examples, see: (a) Wei, M.-X.; Zhang, J.; Ma, F.-L.; Li, M.; Yu, J.-Y.; Luo, W.; Li, X.-Q. Synthesis and biological activities of dithiocarbamates containing 2(5H)-furanone-piperazine. Eur. J. Med. Chem. 2018, 155, 165–170; (b) Quiroga, D.; Becerra, L. D.; Coy-Barrera, E. Ultrasound-Assisted Synthesis, Antifungal Activity against Fusarium oxysporum, and Three-Dimensional Quantitative Structure–Activity Relationship of N,S-Dialkyl Dithiocarbamates Derived from 2-Amino Acids. ACS Omega 2019, 4, 13710–13720; (c) Buac, D.; Schmitt, S.; Ventro, G.; Kona, F. R.; Dou, Q. P. Mini-Rev. Med. Chem. 2012, 12, 1193–1201; (d) Hogarth, G. Metal-dithiocarbamate complexes: chemistry and biological activity. Mini-Rev. Med. Chem. 2012, 12, 1202–1215.
- 3For selected examples, see: (a) Mukherjee, A. K.; Ashare, R. Isothiocyanates in the chemistry of heterocycles. Chem. Rev. 1991, 91, 1–24;
10.1021/cr00001a001 Google Scholar(b) Boas, U.; Gertz, H.; Christensen, J. B.; Heegaard, P. M. H. Facile synthesis of aliphatic isothiocyanates and thioureas on solid phase using peptide coupling reagents. Tetrahedron Lett. 2004, 45, 269–272; (c) Wang, H.; Wang, L.; Shang, J.; Li, X.; Wang, H.; Gui, J.; Lei, A. Fe-catalysed oxidative C–H functionalization/C–S bond formation. Chem. Commun. 2012, 48, 76–78; (d) Yajima, K.; Yamaguchi, K.; Mizuno, N. Facile access to 3,5-symmetrically disubstituted 1,2,4-thiadiazoles through phosphovanadomolybdic acid catalyzed aerobic oxidative dimerization of primary thioamides. Chem. Commun. 2014, 50, 6748–6750.
- 4 Tiwari, V. K.; Singh, A.; Hussain, H. A.; Mishra, B. B.; Tripathi, V. One-Pot Convenient and High Yielding Synthesis of Dithiocarbamates. Monatsh. Chem. 2007, 138, 653–658.
- 5For selected recent examples, see: (a) Wang, Q.; Meng, X.-J.; Tang, H.-T.; Pan, Y.-M.; Duan, W.-G.; He, M.-X. Electrochemically driven α-thiocarbamylation via a dehydrocoupling strategy of β-ketoesters with amines and CS2. Green Chem. 2023, 25, 2572–2576; (b) Halimehjani, A. Z.; Dağalan, Z.; Marjani, Z.; Gündüz, F.; Daştan, A.; Nişancı, B. Catalyst/Metal/Solvent-Free Markovnikov Hydrothiolation of Unactivated Alkenes with Dithiocarbamic Acids. J. Org. Chem. 2024, 89, 5353−536; (c) Kumar, N.; Venkatesh, R.; Kandasamy, Synthesis of functionalized S-benzyl dithiocarbamates from diazo-compounds via multi-component reactions with carbon disulfide and secondary amines. J. Org. Biomol. Chem. 2022, 20, 6766–6770; (d) Halimehjani, A. Z.; Nosood, Y. L. Synthesis of N,S-Heterocycles and Dithiocarbamates by the Reaction of Dithiocarbamic Acids and S-Alkyl Dithiocarbamates with Nitroepoxides. Org. Lett. 2017, 19, 6748–6751; (e) Azizi, N.; Aryanasab, F.; Saidi, M. R. Straightforward and Highly Efficient Catalyst-Free One-Pot Synthesis of Dithiocarbamates under Solvent-Free Conditions. Org. Lett. 2006, 8, 5275–5277; (f) Guntreddi, T.; Vanjari, R.; Singh, K. N. Direct conversion of methylarenes into dithiocarbamates, thioamides and benzyl esters. Tetrahedron, 2014, 70, 3887–3892; (g) Azizi, N.; Khajeh, M.; Hasani, M.; Dezfooli, S. An efficient four-component synthesis of dithiocarbamate derivatives. Tetrahedron Lett. 2013, 54, 5407–5410; (h) Sha, Q.; Wei, Y.-Y. One-pot synthesis of S-alkyl dithiocarbamates via the reaction of N-tosylhydrazones, carbon disulfide and amines. Org. Biomol. Chem. 2013, 11, 5615–5620; (i) Xu, L.-L.; Wang, S.-R.; Sun, J.-Q.; Zhang, R.-J.; Tong, J.; Xu, D.-Z. Facile access to S-aryl/alkyl dithiocarbamates via a three-component reaction under metal-free conditions. Org. Biomol. Chem. 2024, 22, 7702–7706.
- 6(a) Hao, S.; Ye, X.; Zhao, M.; Hu, J.; Wang, N.; Li, J.; Wang, F.; Zhang, M.; Wu, Z. Synthesis of 2-Aryl-2-hydroxyethyl Dithiocarbamates via Regioselective Addition of Tetraalkylthiuram Disulfides to Styrenes under Transition-Metal-Free Conditions. Adv. Synth. Catal. 2020, 362, 5014−5019; (b) Lai, M.; Wu, Z.; Li, S.-J.; Wei, D.; Zhao, M. Regioselective Synthesis of Sulfonyl-Containing Benzyl Dithiocarbamates through Copper-Catalyzed Thiosulfonylation of Styrenes. J. Org. Chem. 2019, 84, 11135−11149; (c) Jiao, J.; Zhang, Z. Copper-Catalyzed Direct C(sp2)–H Sulfuration of Aryl Alkenes by Using Tetraalkylthiuram Disulfides for the Synthesis of Alkenyl Dithiocarbamates. Synthesis 2022, 54, 3588–3594.
- 7(a) Tilles, H. Thiolcarbamates. Preparation and Molar Refractions. J. Am. Chem. Soc. 1959, 81, 714–727; (b) Chaturvedi, D.; Ray, S. An efficient, one-pot, synthesis of dithiocarbamates from the corresponding alcohols using Mitsunobu's reagent. Tetrahedron Lett. 2006, 47, 1307–1309; (c) Walter, W.; Bode, K.-D. Syntheses of Thiocarbamates. Angew. Chem. Int. Ed. Engl. 1967, 6, 281–293.
- 8(a) Wang, Q.; Zhang, C.-L.; Li, Y.-F.; Zhou, Y.-J.; Cui, F.-H.; Jiang, J.-C.; Pan, Y.-M.; Duan, W.-G.; Tang, H.-T. Photoinduced Decarboxylative Thioacylation of N-Hydroxyphthalimide Esters with Tetraalkylthiuram Disulfides. Chem. Eur. J. 2024, e202402716;
(b) Peng, H.-Y.; Dong, Z.-B. Preparation and Microwave Dielectric Properties of Ba3A(V2O7)2 (A = Mg, Zn) Ceramics for ULTCC Applications. Eur. J. Org. Chem. 2019, 7, 949–956.
10.1002/ejoc.201801520 Google Scholar
- 9(a) Guo, H. M.; Wang, J. J.; Xiong, Y.; Wu, X. Visible-Light-Driven Multicomponent Reactions for the Versatile Synthesis of Thioamides by Radical Thiocarbamoylation. Angew. Chem. Int. Ed. 2024, 63, e202409605; (b) Yang, S.-H.; Song, J.-C.; Yang, H.; Zhou, M.-Y.; Wei, Z.-H.; Gao, J.-H.; Dong, D.-Q.; Wang, Z.-L. Visible light induced four component reaction of styrene for the access of thiodifluoroesters. Chin. Chem. Lett. 2023, 34, 108131; (c) Xu, H.; Li, X.; Ma, J.; Zuo, J.; Song, X.; Lv, J.; Yang, D. An electron donor–acceptor photoactivation strategy for the synthesis of S-aryl dithiocarbamates using thianthrenium salts under mild aqueous micellar conditions. Chin. Chem. Lett. 2023, 34, 108403; (d) Lv, Y.; Liu, R.; Ding, H.; Wei, W.; Zhao, X.; He, L. Metal-free visible-light-induced multi-component reactions of α-diazoesters leading to S-alkyl dithiocarbamates. Org. Chem. Front. 2022, 9, 3486–3492; (e) Vishwakarma, R. K.; Kumar, S.; Singh, K. N. Visible-Light-Induced Photocatalytic Synthesis of β-Keto Dithiocarbamates via Difunctionalization of Styrenes. Org. Lett. 2021, 23, 4147−4151; (f) Kumar, M.; Vishwakarma, R.; Preeti, K.; Singh, K. N. Visible-light-mediated C(sp3)–H functionalization of alkyl arylacetates: an easy approach to S-benzyl dithiocarbamate acetates. New J. Chem. 2023, 47, 2412–2416; (g) Guan, Z.-P.; Yang, X.-X.; Zhao, S.-Y.; Yi, Z.-Q.; Wu, Y.-X.; Li, Y.-Y.; Dong, Z.-B. Conversion of Acids to S-Alkyl Dithiocarbamates by Decarboxylative Sulfuration Using Visible-Light Photocatalysis. Org. Lett. 2024, 26, 8323−8328; (h) Wu, Q.-L.; Yang, D.-S. Visible-Light-Driven Multicomponent Reactions for the Versatile Synthesis of Thioamides by Radical Thiocarbamoylation. Chin. J. Org. Chem. 2024, 44, 3571–3573.
- 10For recent reviews, see: (a) Tang, S.; Lin, B.-L.; Tonks, I.; Eagan, J. M.; Ni, X.; Nozaki, K. Sustainable Copolymer Synthesis from Carbon Dioxide and Butadiene. Chem. Rev. 2024, 124, 3590−3607; (b) Cauwenbergh, R.; Goyal, V.; Maiti, R.; Kishore Natte, S. Challenges and recent advancements in the transformation of CO2 into carboxylic acids: straightforward assembly with homogeneous 3d metals. Chem. Soc. Rev. 2022, 51, 9371−9423; (c) Liu, Q.; Wu, L.; Jackstell, R.; Beller, M. Using carbon dioxide as a building block in organic synthesis. Nat. Commun. 2015, 6, 5933; (d) Klankermayer, J.; Wesselbaum, S. Selective Catalytic Synthesis Using the Combination of Carbon Dioxide and Hydrogen: Catalytic Chess at the Interface of Energy and Chemistry. Angew. Chem. Int. Ed. 2016, 55, 7296−7343; (e) Song, Q.-W.; Zhou, Z.-H.; He, L.-N. Efficient, selective and sustainable catalysis of carbon dioxide. Green Chem. 2017, 19, 3707−3728; (f) Yan, S.-S.; Fu, Q.; Liao, L.-L.; Sun, G.-Q.; Ye, J.-H.; Gong, L.; Bo-Xue, Y.-Z.; Yu, D.-G. Transition metal-catalyzed carboxylation of unsaturated substrates with CO2. Coord. Chem. Rev. 2018, 374, 439−463; (g) Artz, J.; Müller, T. E.; Thenert, K.; Kleinekorte, J.; Meys, R.; Sternberg, A.; Bardow, A. Sustainable Conversion of Carbon Dioxide: An Integrated Review of Catalysis and Life Cycle Assessment. Chem. Rev. 2018, 118, 434−504; (h) Yang, Y.; Lee, J.-W. Toward ideal carbon dioxide functionalization. Chem. Sci. 2019, 10, 3905−3926.
- 11(a) Ye, J.-H.; Ju, T.; Huang, H.; Liao, L.-L.; Yu, D.-G. Radical Carboxylative Cyclizations and Carboxylations with CO2. Acc. Chem. Res. 2021, 54, 2518−2531; (b) Xiao, W.; Zhang, J.; Wu, J. Recent Advances in Reactions Involving Carbon Dioxide Radical Anion. ACS Catal. 2023, 13, 15991−16011; (c) Li, X.; Yu, J.; Jaroniec, M.; Chen, X. Cocatalysts for Selective Photoreduction of CO2 into Solar Fuels. Chem. Rev. 2019, 119, 3962−4179; (d) Yeung, C. S. Photoredox Catalysis as a Strategy for CO2 Incorporation: Direct Access to Carboxylic Acids from a Renewable Feedstock. Angew. Chem. Int. Ed. 2019, 58, 5492−5502; (e) Habisreutinger, S. N.; Schmidt-Mende, L.; Stolarczyk, J. K. Photocatalytic Reduction of CO2 on TiO2 and Other Semiconductors. Angew. Chem. Int. Ed. 2013, 52, 7372−7408; (f) Tortajada, A.; Juliá-Hernández, F.; Börjesson, M.; Moragas, T.; Martin, R. Transition-Metal-Catalyzed Carboxylation Reactions with Carbon Dioxide. Angew. Chem. Int. Ed. 2018, 57, 15948−15982; (g) Morris, A. J.; Meyer, G. J.; Fujita, E. Molecular Approaches to the Photocatalytic Reduction of Carbon Dioxide for Solar Fuels. Acc. Chem. Res. 2009, 42, 1983−1994; (h) Cauwenbergh, R.; Das, S. Photochemical reduction of carbon dioxide to formic acid. Green Chem. 2021, 23, 2553−2574.
- 12For selected examples, see: (a) Chaturvedi, D.; Chaturvedi, A. K.; Mishra, V. Carbon Dioxide: Versatile, Cheap and Safe Alternative in the Syntheses of Organic Carbamates. Curr. Org. Chem. 2012, 16, 1609–1635; (b) Ghosh, A. K.; Brindisi, M. Organic Carbamates in Drug Design and Medicinal Chemistry. J. Med. Chem. 2015, 58, 2895–2940; (c) Tobisu, M.; Yasui, K.; Aihara, Y.; Chatani, N. C−O Activation by a Rhodium Bis(N-Heterocyclic Carbene) Catalyst: Aryl Carbamates as Arylating Reagents in Directed C−H Arylation. Angew. Chem. Int. Ed. 2017, 56, 1877–1880; (d) Guo, W.; Gómez, J. E.; Cristòfol, À.; Xie, J.; Kleij, A. W. Catalytic Transformations of Functionalized Cyclic Organic Carbonates. Angew. Chem. Int. Ed. 2018, 57, 13735–13747.
- 13(a) Qin, Y.; Cauwenbergh, R.; Pradhan, S.; Maiti, R.; Franck, P.; Das, S. Straightforward synthesis of functionalized γ-Lactams using impure CO2 stream as the carbon source. Nat. Commun. 2023, 14, 7604; (b) Cheng, R.; Qi, C.; Wang, L.; Xiong, W.; Liu, H.; Jiang, H. Visible light-promoted synthesis of organic carbamates from carbon dioxide under catalyst- and additive-free conditions. Green Chem. 2020, 22, 4890–4895; (c) Guo, Y.; Wei, L.; Wen, Z.; Jiang, H.; Qi, C. Photoredox-catalyzed coupling of aryl sulfonium salts with CO2 and amines to access O-aryl carbamates. Chem. Commun. 2023, 59, 764–767; (d) He, X.; Yao, X.; Cai, S.-F.; Li, H.-R.; He, L.-N. Visible light-driven carbamoyloxylation of the α-C(sp3)–H bond of arylacetones via radical- initiated hydrogen atom transfer. Chem. Commun. 2022, 58, 5845–5848; (e) Wang, L.; Shi, F.; Qi, C.; Xu, W.; Xiong, W.; Kang, B.; Jiang, H. Stereodivergent synthesis of β-iodoenol carbamates with CO2 via photocatalysis. Chem. Sci. 2021, 12, 11821–11830.
- 14For selected reviews, see: (a) Xu, X.; Doyle, M. P. The [3 + 3]-Cycloaddition Alternative for Heterocycle Syntheses: Catalytically Generated Metalloenolcarbenes as Dipolar Adducts. Acc. Chem. Res. 2014, 47, 1396−405; (b) Dong, S.; Liu, X.; Feng, X. Asymmetric Catalytic Rearrangements with α-Diazocarbonyl Compounds. Acc. Chem. Res. 2022, 55, 415−428; (c) Zhao, R.; Shi, L. Reactions between Diazo Compounds and Hypervalent Iodine(III) Reagents. Angew. Chem. Int. Ed. 2020, 59, 12282−12292; (d) Candeias, N. R.; Paterna, R.; Gois, P. M. Homologation Reaction of Ketones with Diazo Compounds. Chem. Rev. 2016, 116, 2937−2981; (e) Mykhailiuk, P. K. 2,2,2-Trifluorodiazoethane (CF3CHN2): A Long Journey since 1943. Chem. Rev. 2020, 120, 12718−12755; (f) Batista, V. F.; Pinto, D. C. G. A.; Silva, A. M. S. Iron: A Worthy Contender in Metal Carbene Chemistry. ACS Catal. 2020, 10, 10096−10116; (g) Xia, Y.; Qiu, D.; Wang, J. Transition-Metal- Catalyzed Cross-Couplings through Carbene Migratory Insertion. Chem. Rev. 2017, 117, 13810−13889; (h) Suleman, M.; Lu, P.; Wang, Y. Recent advances in the synthesis of indole embedded heterocycles with 3-diazoindolin-2-imines. Org. Chem. Front. 2021, 8, 2059−2078; (i) Xiao, Q.; Zhan, Y.; Wang, J. Diazo Compounds and N-Tosylhydrazones: Novel Cross-Coupling Partners in Transition-Metal-Catalyzed Reactions. Acc. Chem. Res. 2013, 46, 236–247.
- 15For selected recent examples, see: (a) Chen, H.; Yang, W.; Zhang, J.; Lu, B.; Wang, X. Divergent Geminal Alkynylation–Allylation and Acylation–Allylation of Carbenes: Evolution and Roles of Two Transition- Metal Catalysts. J. Am. Chem. Soc. 2024, 146, 4727−4740; (b) Yang, D.; Guan, Z.; Peng, Y.; Zhu, S.; Wang, P.; Huang, Z.; Alhumade, H.; Gu, D.; Yi, H.; Lei, A. Electrochemical oxidative difunctionalization of diazo compounds with two different nucleophiles. Nat. Commun. 2023, 14, 1476–1483; (c) Yu, S.; Chang, W.; Hua, R.; Jie, X.; Zhang, M.; Zhao, W.; Chen, J.; Zhang, D.; Qiu, H.; Liang, Y.; Hu, W. An enantioselective four-component reaction via assembling two reaction intermediates. Nat. Commun. 2022, 13, 7088; (d) Wang, G.-Y.; Ge, Z.; Ding, K.; Wang, X. Cooperative Bimetallic Catalysis via One-Metal/Two-Ligands: Mechanistic Insights of Polyfluoroarylation-Allylation of Diazo Compounds. Angew. Chem. Int. Ed. 2023, 62, e202307973; (e) Xu, J.; Ge, Z.; Ding, K.; Wang, X. Rh(II)/Pd(0) Dual-Catalyzed Regio-Divergent Three-Component Propargylic Substitution. JACS Au 2023, 3, 2862–2872; (f) Lu, B.; Liang, X.; Zhang, J.; Wang, Z.; Peng, Q.; Wang, X. Dirhodium(II)/Xantphos-Catalyzed Relay Carbene Insertion and Allylic Alkylation Process: Reaction Development and Mechanistic Insights. J. Am. Chem. Soc. 2021, 143, 11799–11810; (g) Jana, S.; Pei, C. Photochemical Carbene Transfer Reactions of Aryl/Aryl Diazoalkanes— Experiment and Theory. Angew. Chem. Int. Ed. 2021, 60, 13271–13279; (h) Hommelsheim, R.; Guo, Y.; Yang, Z.; Empel, C.; Koenigs, R. M. Blue-Light-Induced Carbene-Transfer Reactions of Diazoalkanes. Angew. Chem. Int. Ed. 2019, 58, 1203–1207; (i) Yuan, W.; Eriksson, L.; Szabo, K. J. Rhodium-Catalyzed Geminal Oxyfluorination and Oxytrifluoro-Methylation of Diazocarbonyl Compounds. Angew. Chem. Int. Ed. 2016, 55, 8410–8415; (j) Hari, D. P.; Waser, J. Copper-Catalyzed Oxy-Alkynylation of Diazo Compounds with Hypervalent Iodine Reagents. J. Am. Chem. Soc. 2016, 138, 2190–2193; (k) Conde, A.; Sabenya, G.; Rodríguez, M.; Postils, V.; Luis, J. M.; Díaz-Requejo, M. M.; Costas, M.; Pérez, P. J. Iron and Manganese Catalysts for the Selective Functionalization of Arene C(sp2)−H Bonds by Carbene Insertion. Angew. Chem. Int. Ed. 2016, 55, 6530–6534; (l) Gao, L.; Kang, B. C.; Ryu, D. H. Catalytic Asymmetric Insertion of Diazoesters into Aryl- CHO Bonds: Highly Enantioselective Construction of Chiral All-Carbon Quaternary Centers. J. Am. Chem. Soc. 2013, 135, 14556–14559; (m) He, X.; Fu, Y.; Xi, R.; Zhang, C.; Lan, K.; Su, Z.; Wang, F.; Feng, X.; Liu, X. Asymmetric Carbene Insertion into Se−S Bonds by Synergistic Rh(II)/Guanidine Catalysis Involving Chalcogen-Bond Assistance. Angew. Chem. Int. Ed. 2025, 64, e202417636.
- 16For selected examples, see: (a) Yi, R.-N.; He, W.-M. Photocatalytic Minisci-type multicomponent reaction for the synthesis of 1-(halo)alkyl-3-heteroaryl bicyclo[1.1.1]pentanes. Chin. Chem. Lett. 2024, 35, 110115; (b) Xu, H.; Li, X.; Wang, Y.; Song, X.; Shi, Y.; Lv, J.; Yang, D. Arylthianthrenium Salts as the Aryl Sources: Visible Light/Copper Catalysis-Enabled Intermolecular Azidosulfonylation of Alkenes. Org. Lett. 2024, 26, 1845–1850; (c) Ji, H.-T.; Wang, K.-L.; Ouyang, W.-T.; Luo, Q.-X.; Li, H.-X.; He, W.-M. Photoinduced, additive- and photosensitizer-free multi-component synthesis of naphthoselenazol-2-amines with air in water. Green Chem. 2023, 25, 7983–7987; (d) Lu, Y.-H.; Wu, C.; Hou, J.-C.; Wu, Z.-L.; Zhou, M.-H.; Huang, X.-J.; He, W.-M. Ferrocene-Mediated Photocatalytic Annulation of N-Sulfonyl Ketimines on a Polycrystalline WSe2 Semiconductor Photocatalyst. ACS Catal. 2023, 13, 13071–13076; (e) Festa, A. A.; Voskressensky, L. G.; Van der Eycken, E. V. Visible light-mediated chemistry of indoles and related heterocycles. Chem. Soc. Rev. 2019, 48, 4401-4423; (f) Chen, J. R.; Hu, X. Q.; Lu, L. Q.; Xiao, W. J. Exploration of Visible-Light Photocatalysis in Heterocycle Synthesis and Functionalization: Reaction Design and Beyond. Acc. Chem. Res. 2016, 49, 1911–1923.
- 17(a) Zhang, Z.; Gevorgyan, V. Visible Light-Induced Reactions of Diazo Compounds and Their Precursors. Chem. Rev. 2024, 124, 7214−7261; (b) Durka, J.; Turkowska, J.; Gryko, D. Lightening Diazo Compounds? ACS Sustainable Chem. Eng. 2021, 9, 8895−8918; (c) Jana, S.; Pei, C.; Empel, R. M. Photochemical Carbene Transfer Reactions of Aryl/Aryl Diazoalkanes—Experiment and Theory. Angew. Chem. Int. Ed. 2021, 60, 13271−13279; (d) Hommelsheim, R.; Guo, Y.; Yang, Z.; Empel, C.; Koenigs, R. M. Blue-Light-Induced Carbene-Transfer Reactions of Diazoalkanes. Angew. Chem. Int. Ed. 2019, 58, 1203−1207; (e) Jurberg, I. D.; Davies, H. M. L. Blue light-promoted photolysis of aryldiazoacetates. Chem. Sci. 2018, 9, 5112–5118.
- 18 Wang, Z.; Herraiz, A. G.; Hoyo, A. M.; Suero, M. G. Generating carbyne equivalents with photoredox catalysis. Nature 2018, 554, 86−91.
- 19(a) He, Q.; Zhang, Q.; Rolka, A. B.; Suero, M. G. Alkoxy Diazomethylation of Alkenes by Photoredox-Catalyzed Oxidative Radical-Polar Crossover. J. Am. Chem. Soc. 2024, 146, 12294−12299; (b) Wu, F.-P.; Chintawar, C. C.; Lalisse, R.; Mukherjee, P.; Dutta, S.; Tyler, J.; Daniliuc, C. G.; Gutierrez, O.; Glorius, F. Ring expansion of indene by photoredox-enabled functionalized carbon-atom insertion. Nat. Catal. 2024, 7, 242−251; (c) Li, X.; Golz, C.; Alcarazo, M. α-Diazo Sulfonium Triflates: Synthesis, Structure, and Application to the Synthesis of 1-(Dialkylamino)-1,2,3-triazoles. Angew. Chem. Int. Ed. 2021, 60, 6943–6948; (d) Su, Y.-L.; Dong, K.; Zheng, H.; Doyle, M. P. Generation of Diazomethyl Radicals by Hydrogen Atom Abstraction and Their Cycloaddition with Alkenes. Angew. Chem. Int. Ed. 2021, 60, 18484–18488; (e) He, M.-Y.; Tang, X.; Wu, H.-Y.; Nie, J.; Ma, J.-A.; Zhang, F.-G. Electron Donor–Acceptor Complex Enabled Radical Cyclization of α-Diazodifluoroethyl Sulfonium Salt with Unactivated Alkynes. Org. Lett. 2023, 25, 9041–9046; (f) Wen, J.; Zhao, W.; Gao, X.; Ren, X.; Dong, C.; Wang, C.; Li, L.; Li, J. Synthesis of [1,2,3]Triazolo-[1,5-a]quinoxalin-4(5H)-ones through Photoredox-Catalyzed [3 + 2] Cyclization Reactions with Hypervalent Iodine(III) Reagents. J. Org. Chem. 2022, 87, 4415–4423; (g) Zhao, W. W.; Shao, Y.-C.; Wang, A.-N.; Huang, J.-L.; He, C.-Y.; Cui, B.-D.; Wan, N.-W.; Chen, Y.-Z.; Han, W.-Y. Diazotrifluoroethyl Radical: A CF3-Containing Building Block in [3 + 2] Cycloaddition. Org. Lett. 2021, 23, 9256–9261; (h) Dong, J.-Y.; Wang, H.; Mao, S.; Wang, X.; Zhou, M.-D.; Li, L. Visible Light-Induced [3+2] Cyclization Reactions of Hydrazones with Hypervalent Iodine Diazo Reagents for the Synthesis of 1-Amino-1,2,3-Triazoles. Adv. Synth. Catal. 2021, 363, 2133−2139; (i) Li, J.; Lu, X.-C.; Xu, Y.; Wen, J.-X.; Hou, G.-Q.; Liu, L. Photoredox Catalysis Enables Decarboxylative Cyclization with Hypervalent Iodine(III) Reagents: Access to 2,5-Disubstituted 1,3,4-Oxadiazoles. Org. Lett. 2020, 22, 9621–9626; (j) Huang, M.; Wang, G.; Li, H.; Zou, Z.; Jia, X.; Karotsis, G.; Pan, Y.; Zhang, W.; Ma, J.; Wang, Y. EDA complex-mediated [3 + 2] cyclization for the synthesis of CF3-oxadiazoles. Green Chem. 2025, 27, 413-419; (k) Zeng, Y.; Zheng, X.; Shen, L.; Jing, Y.; Chen, S.; Luo, Z.; Ke, Z.; Xie, H.; Liu, J.; Jiang, H.; Zeng, W. Oxydiazomethylation of Alkenes via Photoredox Catalysis. Chem. Eur. J. 2025, 31, e202403509; (l) Timmann, S.; Alcarazo, M. α-Diazo-λ3-iodanes and α-diazo sulfonium salts: the umpolung of diazo compounds. Chem. Commun. 2023, 59, 8032–8042; (m) Timmann, S.; Wu, T.-H.; Golz, C.; Alcarazo, M. Reactivity of α-diazo sulfonium salts: rhodium-catalysed ring expansion of indenes to naphthalenes. Chem. Sci. 2024, 15, 5938–5943.
- 20For selected examples, see: (a) Xu, X.-C.; Sang, Y.; Yang, M.; He, B.-W.; Zhang, Y.-C.; Yuan, H.-Y.; Zhao, Y.-L. A visible light-induced photoredox-catalyzed assembly-point di/trifunctionalization of diazomethyl radicals. Org. Chem. Front. 2024, 11, 5502–5510; (b) Xu, X.-C.; Gong, Y.; Wang, J.; Yuan, Y.-R.; Zhao, Y.-L. DBU-Promoted Tandem Cyclization of Ynones and Diazo Compounds: Direct Synthesis of Eight- Membered Cyclic Ethers. Org. Lett. 2023, 25, 5750–5755; (c) Liang, Y. X.; Wang, J.; Xu, X.-C.; Gong, Y.; Zhao, Y.-L. Lewis Acid Mediated Conjugate Addition of Isocyanides to β-Hydroxy-α-diazo Carbonyls: Synthesis of β-Carboxamido-α-diazo Carbonyl Compounds. Org. Lett. 2023, 25, 200–204; (d) Zhang, L.; Yang, M.; Gong, Y.; Wang, J.; Zhao, Y.-L. n-BuLi-promoted nucleophilic addition of unactivated C(sp3)–H bonds to diazo compounds as N-terminal electrophiles: efficient synthesis of hydrazine derivatives. Org. Chem. Front. 2023, 10, 499–505; (e) Zhang, L.; Liu, T.; Wang, Y. M.; Chen, J.; Zhao, Y.-L. Rhodium- Catalyzed Coupling–Cyclization of Alkenyldiazoacetates with o-Alkenyl Arylisocyanides: A General Route to Carbazoles. Org. Lett. 2019, 21, 2973–2977; (f) Li, L.; Chen, J.-J.; Li, Y.-J.; Bu, X.-B.; Liu, Q.; Zhao, Y.-L. Activation of α-Diazocarbonyls by Organic Catalysts: Diazo Group Acting as a Strong N-Terminal Electrophile. Angew. Chem. Int. Ed. 2015, 54, 12107–12111; (g) Xu, X.-C.; Wu, D.-N.; Liang, Y.-X.; Yang, M.; Yuan, H.-Y.; Zhao, Y.-L. Visible Light-Induced Coupling Cyclization Reaction of α-Diazosulfonium Triflates with α-Oxocarboxylic Acids or Alkynes. J. Org. Chem. 2022, 87, 16604–16616; (h) Yu, Y.; Zhang, Y.; Wang, Z.; Liang, Y.-X.; Zhao, Y.-L. A rhodium-catalyzed three-component reaction of arylisocyanides, trifluorodiazoethane, and activated methylene isocyanides or azomethine ylides: an efficient synthesis of trifluoroethyl-substituted imidazoles. Org. Chem. Front. 2019, 6, 3657–3662; (i) Liang, Y.-X.; Meng, X.-H.; Yang, M.; Haroon, M.; Zhao, Y.-L. Zn(OAc)2-catalyzed tandem cyclization of isocyanides, α-diazoketones, and anhydrides: a general route to polysubstituted maleimides. Chem. Commun. 2019, 55, 12519–12522.
- 21(a) Yang, M.; Meng, Y.-X.; Mehfooz, H.; Zhao, Y.-L. Visible light-promoted [3+2] cyclization reaction of vinyl azides with perfluoroalkyl- substituted-imidoyl sulfoxonium ylides. Chem. Commun. 2024, 60, 5407–5410; (b) Liang, Y.-X.; Gong, Y.; Xu, X.-C.; Yang, M.; Zhao, Y.-L. Visible light-induced radical cyclization of o-alkenyl aromatic isocyanides with thioethers: direct synthesis of 2-thioquinolines. Org. Chem. Front. 2024, 11, 2033–2039; (c) Yang, M.; Wang, X.-Y.; Wang, J.; Zhao, Y.-L. Visible Light-Induced [3+2] Annulation Reaction of Alkenes with Vinyl Azides: Direct Synthesis of Functionalized Pyrroles. Chin. J. Chem. 2024, 42, 151–156; (d) Yang, Z.-X.; Xu, X.-C.; He, B.-W.; Meng, Y.-X.; Zhao, Y.-L. Dual Photoredox/Copper-Catalyzed Three-Component Alkylcyanation of Alkenes and 1,4-Alkylcyanation of 1,3-Enynes Employing Sulfoxonium Ylides as the Carbon Radical Precursors. Org. Lett. 2024, 26, 10576–10582.
- 22 CCDC 2394065 (3ar) contains the supplementary crystallographic data for this paper. These data can be obtained free of charge from The Cambridge Crystallographic Data Centre via www.ddcd.cam.ac.uk/data_request/cif.




