Chiral Ag-Complex Catalyzed Enantioselective α-Functionalization of Cyclic Azomethine Ylides with Concomitant Remote-Controlled Asymmetric Desymmetrization of N-Arylmaleimides and Cyclopentene-1,3-diones
Ji-Hong Liu
Innovation Research Center of Chiral Drugs, Institute for Advanced Study, Chengdu University, Chengdu, Sichuan, 610106 China
National Engineering Research Center of Chiral Drugs, Chengdu Institute of Organic Chemistry, Chinese Academy of Sciences, Chengdu, Sichuan, 610041 China
Search for more papers by this authorJian-Mei Wang
Innovation Research Center of Chiral Drugs, Institute for Advanced Study, Chengdu University, Chengdu, Sichuan, 610106 China
National Engineering Research Center of Chiral Drugs, Chengdu Institute of Organic Chemistry, Chinese Academy of Sciences, Chengdu, Sichuan, 610041 China
Search for more papers by this authorYan-Ping Zhang
Innovation Research Center of Chiral Drugs, Institute for Advanced Study, Chengdu University, Chengdu, Sichuan, 610106 China
Search for more papers by this authorZhen-Hua Wang
Innovation Research Center of Chiral Drugs, Institute for Advanced Study, Chengdu University, Chengdu, Sichuan, 610106 China
Search for more papers by this authorLei Yang
Innovation Research Center of Chiral Drugs, Institute for Advanced Study, Chengdu University, Chengdu, Sichuan, 610106 China
Search for more papers by this authorJian-Qiang Zhao
Innovation Research Center of Chiral Drugs, Institute for Advanced Study, Chengdu University, Chengdu, Sichuan, 610106 China
Search for more papers by this authorCorresponding Author
Ming-Qiang Zhou
National Engineering Research Center of Chiral Drugs, Chengdu Institute of Organic Chemistry, Chinese Academy of Sciences, Chengdu, Sichuan, 610041 China
E-mail: [email protected] (W.-C. Yuan); [email protected] (Y. You); [email protected] (M.-Q. Zhou)Search for more papers by this authorCorresponding Author
Yong You
Innovation Research Center of Chiral Drugs, Institute for Advanced Study, Chengdu University, Chengdu, Sichuan, 610106 China
E-mail: [email protected] (W.-C. Yuan); [email protected] (Y. You); [email protected] (M.-Q. Zhou)Search for more papers by this authorCorresponding Author
Wei-Cheng Yuan
Innovation Research Center of Chiral Drugs, Institute for Advanced Study, Chengdu University, Chengdu, Sichuan, 610106 China
E-mail: [email protected] (W.-C. Yuan); [email protected] (Y. You); [email protected] (M.-Q. Zhou)Search for more papers by this authorJi-Hong Liu
Innovation Research Center of Chiral Drugs, Institute for Advanced Study, Chengdu University, Chengdu, Sichuan, 610106 China
National Engineering Research Center of Chiral Drugs, Chengdu Institute of Organic Chemistry, Chinese Academy of Sciences, Chengdu, Sichuan, 610041 China
Search for more papers by this authorJian-Mei Wang
Innovation Research Center of Chiral Drugs, Institute for Advanced Study, Chengdu University, Chengdu, Sichuan, 610106 China
National Engineering Research Center of Chiral Drugs, Chengdu Institute of Organic Chemistry, Chinese Academy of Sciences, Chengdu, Sichuan, 610041 China
Search for more papers by this authorYan-Ping Zhang
Innovation Research Center of Chiral Drugs, Institute for Advanced Study, Chengdu University, Chengdu, Sichuan, 610106 China
Search for more papers by this authorZhen-Hua Wang
Innovation Research Center of Chiral Drugs, Institute for Advanced Study, Chengdu University, Chengdu, Sichuan, 610106 China
Search for more papers by this authorLei Yang
Innovation Research Center of Chiral Drugs, Institute for Advanced Study, Chengdu University, Chengdu, Sichuan, 610106 China
Search for more papers by this authorJian-Qiang Zhao
Innovation Research Center of Chiral Drugs, Institute for Advanced Study, Chengdu University, Chengdu, Sichuan, 610106 China
Search for more papers by this authorCorresponding Author
Ming-Qiang Zhou
National Engineering Research Center of Chiral Drugs, Chengdu Institute of Organic Chemistry, Chinese Academy of Sciences, Chengdu, Sichuan, 610041 China
E-mail: [email protected] (W.-C. Yuan); [email protected] (Y. You); [email protected] (M.-Q. Zhou)Search for more papers by this authorCorresponding Author
Yong You
Innovation Research Center of Chiral Drugs, Institute for Advanced Study, Chengdu University, Chengdu, Sichuan, 610106 China
E-mail: [email protected] (W.-C. Yuan); [email protected] (Y. You); [email protected] (M.-Q. Zhou)Search for more papers by this authorCorresponding Author
Wei-Cheng Yuan
Innovation Research Center of Chiral Drugs, Institute for Advanced Study, Chengdu University, Chengdu, Sichuan, 610106 China
E-mail: [email protected] (W.-C. Yuan); [email protected] (Y. You); [email protected] (M.-Q. Zhou)Search for more papers by this authorComprehensive Summary
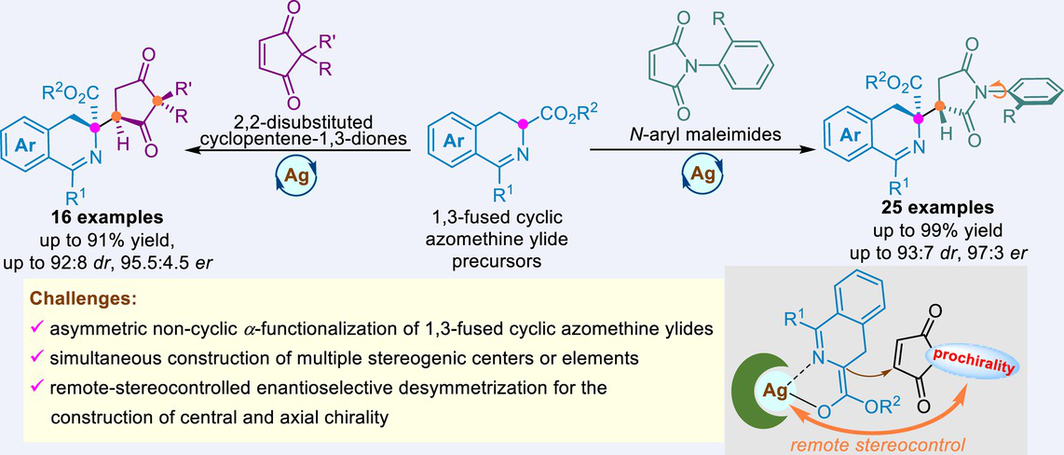
The asymmetric cycloaddition reactions of 1,3-fused cyclic azomethine ylides have been extensively studied, but the non-cyclic α-functionalization of these compounds remains unexplored. Herein, an efficient combination of the catalytic enantioselective non-cyclic α-functionalization of 1,3-fused cyclic azomethine ylides and the remote-controlled asymmetric desymmetrization of N-arylmaleimides and cyclopentene-1,3-diones has been achieved with a catalyst system consisting of a chiral P,N-ferrocene ligand and AgNO2. This reaction allowed for the synthesis of a series of enantioenriched 3,4-dihydroisoquinoline derivatives bearing multiple stereogenic elements/centers with good yields and stereoselectivities. The practicality of this method was demonstrated by gram-scale synthesis and derivatizations of the products.
Supporting Information
| Filename | Description |
|---|---|
| cjoc70076-sup-0001-Supinfo.pdfPDF document, 11.3 MB |
Appendix S1: Supporting Information |
Please note: The publisher is not responsible for the content or functionality of any supporting information supplied by the authors. Any queries (other than missing content) should be directed to the corresponding author for the article.
References
- 1For recent reviews, see: (a) Adrio, J.; Carretero, J. C. Stereochemical diversity in pyrrolidine synthesis by catalytic asymmetric 1,3-dipolar cycloaddition of azomethine ylides. Chem. Commun. 2019, 55, 11979–11991; (b) Adrio, J.; Carretero, J. C. Recent advances in the catalytic asymmetric 1,3-dipolar cycloaddition of azomethine ylides. Chem. Commun. 2014, 50, 12434–12446; (c) Hashimoto, T.; Maruoka, K. Recent advances of catalytic asymmetric 1,3-dipolar cycloadditions. Chem. Rev. 2015, 115, 5366–5412.
- 2For selected reviews, see: (a) Döndas, H. A.; de Gracia Retamosa, M.; Sansano, J. M. Current trends towards the synthesis of bioactive heterocycles and natural products using 1,3-dipolar cycloadditions (1,3-DC) with azomethine ylides. Synthesis 2017, 49, 2819–2851;
(b) Liang, W.; Lu, X.; Hu, Y.; Wang, Z.; Tao, H.; Wang, C. Recent advances in metallated azomethine ylides for the synthesis of chiral unnatural α-amino acids. Chin. J. Org. Chem. 2019, 39, 2119–2130;
10.6023/cjoc201904060 Google Scholar(c) Arrastia, I.; Arrieta, A.; Cossío, F. P. Application of 1,3-Dipolar Reactions between Azomethine Ylides and Alkenes to the Synthesis of Catalysts and Biologically Active Compounds. Eur. J. Org. Chem. 2018, 2018, 5889–5904.
- 3For applications in natural products, see: (a) Monn, J. A.; Thurkauf, A.; Mattson, M. V.; Jacobson, A. E.; Rice, K. C. Synthesis and structure- activity relationship of C5-substituted analogs of (.+-.)-10,11-dihydro- 5H-dibenzo [a,d] cyclohepten-5,10-imine [(.+-.)-desmethyl-MK801]: ligands for the NMDA receptor-coupled phencyclidine binding site. J. Med. Chem. 1990, 33, 1069–1076; (b) Grynkiewicz, G.; Gadzikowska, M. Tropane alkaloids as medicinally useful natural products and their synthetic derivatives as new drugs. Pharmacol. Rep. 2008, 60, 439–462; (c) Afewerki, S.; Wang, J.-X.; Liao, W.-W.; Córdova, A. The chemical synthesis and applications of tropane alkaloids. Alkaloids: Chem. Biol. 2019, 81, 151–233. For selected synthetic methods of benzotropane skeletons, see: (d) Schultz, D. M.; Wolfe, J. P. Intramolecular alkene carboamination reactions for the synthesis of enantiomerically enriched tropane derivatives. Org. Lett. 2011, 13, 2962–2965; (e) Tan, T.-D.; Zhu, X.-Q.; Bu, H.-Z.; Deng, G.; Chen, Y.-B.; Liu, R.-S.; Ye, L.-W. Copper-Catalyzed Cascade Cyclization of Indolyl Homopropargyl Amides: Stereospecific Construction of Bridged Aza-[n.2.1] Skeletons. Angew. Chem. Int. Ed. 2019, 58, 9632–9639; (f) Xu, J.-H.; Zheng, S.-C.; Zhang, J.-W.; Liu, X.-Y.; Tan, B. Construction of tropane derivatives by the organocatalytic asymmetric dearomatization of isoquinolines. Angew. Chem. Int. Ed. 2016, 55, 11834–11839.
- 4
R. Narayan; J. O. Bauer; C. Strohmann; A. P. Antonchick; H. Waldmann Catalytic enantioselective synthesis of functionalized tropanes reveals novel inhibitors of hedgehog signaling. Angew. Chem. Int. Ed. 2013, 52, 12892–12896; Angew. Chem. Int. Ed. 2013, 125, 13130–13134.
10.1002/ange.201307392 Google Scholar
- 5 Wang, Z.; Wang, D.-C.; Xie, M.-S.; Qu, G.-R. Guo, H.-M. Enantioselective synthesis of fused polycyclic tropanes via dearomative [3+2] cycloaddition reactions of 2-nitrobenzofurans. Org. Lett. 2019, 22, 164–167.
- 6(a) Tan, J.-P.; Li, X.; Chen, Y.; Rong, X.Zhu, L.; Jiang, C.; Xiao, K.; Wang, T. Highly stereoselective construction of polycyclic benzofused tropane scaffolds and their latent bioactivities: Bifunctional phosphonium salt-enabled cyclodearomatization process. Sci. China Chem. 2020, 63, 1091–1099; (b) Chen, Y.; He, J.; Zhuang, C.; Liu, Z.; Xiao, K.; Su, Z.; Ren, X.; Wang, T. Synergistic Catalysis between a Dipeptide Phosphonium Salt and a Metal-Based Lewis Acid for Asymmetric Synthesis of N-Bridged [3.2.1] Ring Systems. Angew. Chem. Int. Ed. 2022, 61, e202207334; (c) Tan, J.-P.; Li, K.; Shen, B.; Zhuang, C.; Liu, Z.; Xiao, K.; Yu, P.; Yi, B.; Ren, X.; Wang, T. Asymmetric synthesis of N-bridged [3.3.1] ring systems by phosphonium salt/Lewis acid relay catalysis. Nat. Commun. 2022, 13, 357–368.
- 7(a) Wang, M.; Feng, M.; Tang, B.; Jiang, X. Recent advances of desymmetrization protocol applied in natural product total synthesis. Tetrahedron Lett. 2014, 55, 7147–7155;
(b) Merad, J.; Candy, M.; Pons, J. M.; Bressy, C. Catalytic enantioselective desymmetrization of meso compounds in total synthesis of natural products: towards an economy of chiral reagents. Synthesis 2017, 49, 1938–1954;
(c) Horwitz, M. A.; Johnson, J. S. Local desymmetrization through diastereotopic group selection: An enabling strategy for natural product synthesis. Eur. J. Org. Chem. 2017, 11, 1381–1390;
10.1002/ejoc.201601481 Google Scholar(d) Inai, M.; Asakawa, T.; Kan, T. Total synthesis of natural products using a desymmetrization strategy. Tetrahedron Lett. 2018, 59, 1343–1347.
- 8For recent reviews, see: (a) Borissov, A.; Davies, T. Q.; Ellis, S. R.; Fleming, T. A.; Richardson, M. S. W.; Dixon, D. J. Organocatalytic enantioselective desymmetrisation. Chem. Soc. Rev., 2016, 45, 5474–5540; (b) Shu, T.; Cossy, J. Asymmetric desymmetrization of alkene-, alkyne-and allene-tethered cyclohexadienones using transition metal catalysis. Chem. Soc. Rev. 2021, 50, 658–666; (c) Nájera, C.; Foubelo, F.; Sansano, J. M.; Yus, M. Enantioselective desymmetrization reactions in asymmetric catalysis. Tetrahedron 2022, 106, 132629.
- 9For recent examples, see: (a) Liu, H.-C.; Tao, H.-Y.; Cong, H.; Wang, C.-J. Silver (I)-catalyzed atroposelective desymmetrization of N-arylmaleimide via 1,3-dipolar cycloaddition of azomethine ylides: Access to octahydropyrrolo [3,4-c] pyrrole derivatives. J. Org. Chem. 2016, 81, 3752−3760; (b) Wu, Y.; Xu, B.; Liu, B.; Zhang, Z.-M.; Liu, Y. A new trifluoromethylated sulfonamide phosphine ligand for Ag (I)-catalyzed enantioselective [3+2] cycloaddition of azomethine ylides. Org. Biomol. Chem. 2019, 17, 1395–1401; (c) Mondal, S.; Mukherjee, S. Catalytic Generation of Remote C–N Axial Chirality through Atroposelective de novo Arene Construction. Org. Lett. 2022, 24, 8300−8304; (d) Barik, S.; Das, R. C.; Balanna, K.; Biju, A. T. Kinetic resolution approach to the synthesis of C–N axially chiral N-aryl aminomaleimides via NHC-catalyzed [3+3] annulation. Org. Lett. 2022, 24, 5456−5461; (e) Wang, H.; Wei, Y.; Li, Y.; Long, S.; Sun, L.-J.; Li, S.; Lin, Y.-W. Phosphine-Catalyzed Atroposelective Formal [3+2] Cycloaddition Desymmetrization of N-Arylmaleimides. Org. Lett. 2022, 24, 6494−6498; (f) Zhang, S.; Luo, Z.-H.; Wang, W.-T.; Qian, L.; Liao, J.-Y. Simultaneous Construction of C–N Axial and Central Chirality via Silver-Catalyzed Desymmetrizative [3+2] Cycloaddition of Prochiral N-Aryl Maleimides with Activated Isocyanides. Org. Lett. 2022, 24, 4645−4649; (g) Barday, M.; Rodrigues, J.; Bouillac, P.; Rodriguez, J.; Amatore, M. N-Heterocyclic Carbene Control over Multiple Stereogenicities: Atroposelective Synthesis of Axially Chiral Phthalimides. Adv. Synth. Catal. 2023, 365, 148–155; (h) Hou, J.; Hao, W.; Chen, Y.; Wang, Z.; Yao, W. Phosphine-Catalyzed Stereospecific and Enantioselective Desymmetrizative [3+2] Cycloaddition of MBH Carbonates and N-(2-tert-Butylphenyl) maleimides. J. Org. Chem. 2024, 89, 9068−9077.
- 10(a) Duan, W. L.; Imazaki, Y.; Shintani, R.; Hayashi, T. Asymmetric construction of chiral C–N axes through rhodium-catalyzed 1,4-addition. Tetrahedron 2007, 63, 8529–8536;
(b) Iorio, N. D.; Righi, P.; Mazzanti, A.; Mazzanti, M.; Ciogli, A.; Bencivenni, G. Remote control of axial chirality: aminocatalytic desymmetrization of N-arylmaleimides via vinylogous Michael addition. J. Am. Chem. Soc. 2014, 136, 10250–10253;
(c) Zhang, J.; Zhang, Y.; Lin, L.; Yao, Q.; Liu, X.; Feng, X. Catalytic asymmetric desymmetrization of N-arylmaleimides: efficient construction of both atom chirality and axial chirality. Chem. Commun. 2015, 51, 10554−10557;
(d) Iorio, N. D.; Champavert, F.; Erice, A.; Righi, P.; Mazzanti, A. Targeting remote axial chirality control of N-(2-tert-butylphenyl) succinimides by means of Michael addition type reactions. Tetrahedron 2016, 72, 5191−5201;
10.1016/j.tet.2016.02.052 Google Scholar(e) Iorio, N. D.; Soprani, L.; Crotti, S.; Marotta, E.; Mazzanti, A.; Righi, P.; Bencivenni, G. Targeting remote axial chirality control of N-(2-tert-butylphenyl) succinimides by means of Michael addition type reactions. Synthesis 2017, 49, 1519–1530; (f) Barik, S.; Shee, S.; Das, S.; Gonnade, R. G.; Jindal, G.; Mukherjee, S.; Biju, A. T. NHC-catalyzed desymmetrization of n-aryl maleimides leading to the atroposelective synthesis of N-Aryl succinimides. Angew. Chem. Int. Ed. 2021, 60, 12264–12268; (g) Hans, A. C.; Becker, P. M.; Haußmann, J.; Suhr, S.; Wanner, D. M.; Lederer, V.; Willig, F.; Frey, W.; Sarkar, B.; Kästner, J.; Peters, R. A practical and robust zwitterionic cooperative Lewis acid/acetate/ benzimidazolium catalyst for direct 1,4-additions. Angew. Chem. Int. Ed. 2023, 62, e202217519.
- 11 Manna, M. S.; Mukherjee, S. Catalytic asymmetric desymmetrization approaches to enantioenriched cyclopentanes. Org. Biomol. Chem. 2015, 13, 18–24.
- 12For selected reviews, see: (a) Mehta, G.; Srikrishna, A. Synthesis of polyquinane natural products: An update. Chem. Rev. 1997, 97, 671–720; (b) Biellmann, J. F. Enantiomeric steroids: synthesis, physical, and biological properties. Chem. Rev. 2003, 103, 2019; (c) Silva, L. F. Jr. Construction of cyclopentyl units by ring contraction reactions. Tetrahedron. 2002, 58, 9137–9161; (d) Faulkner, D. J. Modified Cyclic Hexapeptides from the Philippines Ascidian Lissoclinum b istratum. Nat. Prod. Rep. 2002, 19, 1; (e) Das, S.; Chandrasekhar, S.; Yadav, J. S.; Grée, R. Recent developments in the synthesis of prostaglandins and analogues. Chem. Rev. 2007, 107, 3286–3337.
- 13 Wang, Z.-H.; Liu, J.-H.; Zhang, Y.-P.; Zhao, J.-Q.; You, Y.; Zhou, M.-Q.; Han, W.-Y.; Yuan, W.-C. Cu-catalyzed asymmetric 1,3-dipolar cycloaddition of N-2,2,2-trifluoroethylisatin ketimines enables the desymmetrization of N-arylmaleimides: access to enantioenriched F3C-containing octahydropyrrolo [3,4-c] pyrroles. Org. Lett. 2022, 24, 4052–4057.
- 14CCDC-2428597 (3a) and 2428598 (5k) contains the supplementary crystallo-graphic data for this paper. These data can be obtained free of charge from The Cambridge Crystallographic Data Centre via www.ccdc.cam.ac.uk/data_request/cif.
- 15 Das, T.; Saha, P.; Singh, V. K. Silver(I)−Ferrophox Catalyzed Enantioselective Desymmetrization of Cyclopentenedione: Synthesis of Highly Substituted Bicyclic Pyrrolidines, Org. Lett. 2015, 17, 5088−5091.




