Achieving Efficient TTA-UC Both in Organogels and Solvent-Free Dry Gels by Co-assembling Annihilator with Gelators
Jiao Chen
Key Laboratory of Green Chemistry & Technology, College of Chemistry, Sichuan University, 29 Wangjiang Road, Chengdu, Sichuan, 610064 China
Search for more papers by this authorPinyou Wang
Key Laboratory of Green Chemistry & Technology, College of Chemistry, Sichuan University, 29 Wangjiang Road, Chengdu, Sichuan, 610064 China
Search for more papers by this authorJinbo Liu
Key Laboratory of Green Chemistry & Technology, College of Chemistry, Sichuan University, 29 Wangjiang Road, Chengdu, Sichuan, 610064 China
Search for more papers by this authorCorresponding Author
Cheng Yang
Key Laboratory of Green Chemistry & Technology, College of Chemistry, Sichuan University, 29 Wangjiang Road, Chengdu, Sichuan, 610064 China
E-mail: [email protected]; [email protected]Search for more papers by this authorCorresponding Author
Wanhua Wu
Key Laboratory of Green Chemistry & Technology, College of Chemistry, Sichuan University, 29 Wangjiang Road, Chengdu, Sichuan, 610064 China
E-mail: [email protected]; [email protected]Search for more papers by this authorJiao Chen
Key Laboratory of Green Chemistry & Technology, College of Chemistry, Sichuan University, 29 Wangjiang Road, Chengdu, Sichuan, 610064 China
Search for more papers by this authorPinyou Wang
Key Laboratory of Green Chemistry & Technology, College of Chemistry, Sichuan University, 29 Wangjiang Road, Chengdu, Sichuan, 610064 China
Search for more papers by this authorJinbo Liu
Key Laboratory of Green Chemistry & Technology, College of Chemistry, Sichuan University, 29 Wangjiang Road, Chengdu, Sichuan, 610064 China
Search for more papers by this authorCorresponding Author
Cheng Yang
Key Laboratory of Green Chemistry & Technology, College of Chemistry, Sichuan University, 29 Wangjiang Road, Chengdu, Sichuan, 610064 China
E-mail: [email protected]; [email protected]Search for more papers by this authorCorresponding Author
Wanhua Wu
Key Laboratory of Green Chemistry & Technology, College of Chemistry, Sichuan University, 29 Wangjiang Road, Chengdu, Sichuan, 610064 China
E-mail: [email protected]; [email protected]Search for more papers by this authorComprehensive Summary
Supramolecular organogels were highly promising matrices for triplet–triplet annihilation-based upconversion (TTA-UC), but the dispersion and diffusion of the UC components were greatly relied on the microscopically interconnected solution phase in gels. Herein, 12-hydroxystearic acid (CA) and its derivatives with different alkyl chain (CA3, CA4, CA6 and CA8) were synthesized as low-molecular-weight gelators (LMMGs), and D-1 with CA attached on DPA unit was synthesized as annihilator. It was found that by co-assembling D-1 with the LMMGs, the DPA units were uniformly dispersed in the gel network regardless of whether there was a solvent or not. By chemically tuning LMMGs to optimize the morphologies of organogels, the DPA units were orderly arranged in the gel network, and showing efficient UC emission in CA, CA3, and CA8 which showed more regular morphologies. UC quantum yield of up to 13.4% (out of 50% maximum) was achieved in CA3 organogel. Moreover, when all solvents were removed from the organogels, D-1 also showed significant UC emissions, which was more than 6-fold higher than that of DPA, indicating that co-assembling the annihilator with the matrix to achieve an order arrangement presented an efficient strategy towards efficient TTA-UC in solid state.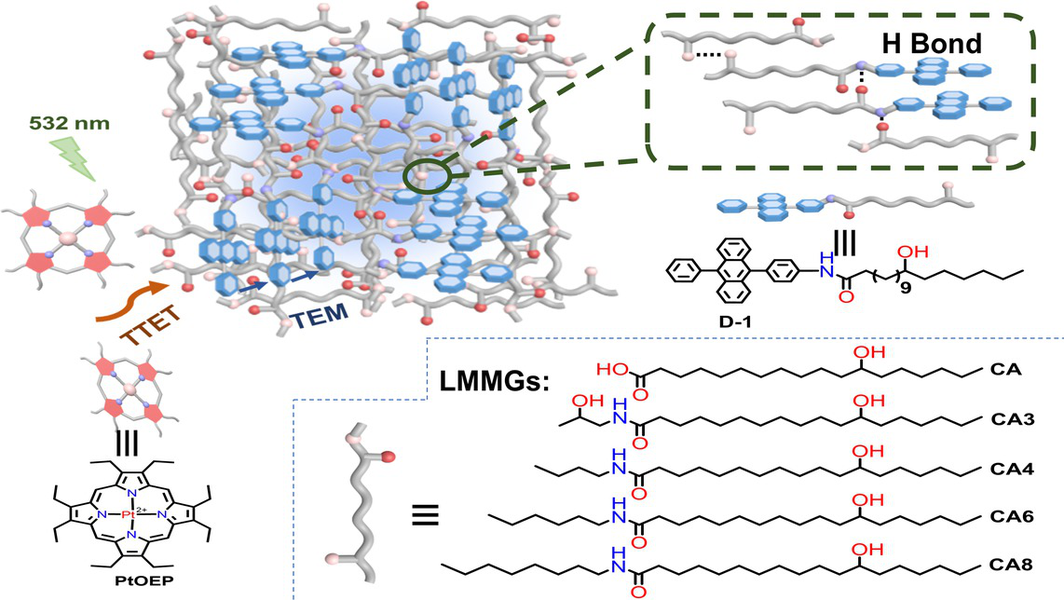
Supporting Information
| Filename | Description |
|---|---|
| cjoc70073-sup-0001-Supinfo.pdfPDF document, 3.2 MB |
Appendix S1: supporting Information |
Please note: The publisher is not responsible for the content or functionality of any supporting information supplied by the authors. Any queries (other than missing content) should be directed to the corresponding author for the article.
References
- 1 Rao, M.; Kanagaraj, K.; Fan, C. Y.; Ji, J. C.; Xiao, C.; Wei, X. Q.; Wu, W. H.; Yang, C. Photocatalytic Supramolecular Enantiodifferentiating Dimerization of 2-Anthracenecarboxylic Acid through Triplet–Triplet Annihilation. Org. Lett. 2018, 20, 1680–1683.
- 2 Liu, S. S.; Liu, H. Y.; Hu, Y. J.; Zhao, C. Y.; Huang, H. B.; Yu, G. Y.; Li, Z.; Liu, Z. B.; Chen, Y. L.; Li, X. Y. Boosting photocatalytic hydrogen evolution via triplet–triplet annihilation upconversion. Chem. Eng. J. 2023, 452, 139203.
- 3 Häring, M.; Abramov, A.; Okumura, K.; Ghosh, I.; König, B.; Yanai, N.; Kimizuka, N.; Díaz Díaz, D. Air-Sensitive Photoredox Catalysis Performed under Aerobic Conditions in Gel Networks. J. Org. Chem. 2018, 83, 7928–7938.
- 4 Rao, M.; Wu, W. H.; Yang, C. Effects of Temperature and Host Concentration on the Supramolecular Enantiodifferentiating [4 + 4] Photodimerization of 2-Anthracenecarboxylate through Triplet-Triplet Annihilation Catalyzed by Pt-Modified Cyclodextrins. Molecules 2019, 24, 1502.
- 5 Rao, M.; Fan, C. Y.; Ji, J. C.; Liang, W. T.; Wei, L. L.; Zhang, D. J.; Yan, Z. Q.; Wu, W. H.; Yang, C. Catalytic Chiral Photochemistry Sensitized by Chiral Hosts-Grafted Upconverted Nanoparticles. ACS Appl. Mater. Interfaces 2022, 14, 21453–21460.
- 6 Peng, P. F.; Wu, N.; Ye, L. X.; Jiang, F. L.; Feng, W.; Li, F. Y.; Liu, Y. S.; Hong, M. C. Biodegradable Inorganic Upconversion Nanocrystals for In Vivo Applications. ACS Nano 2020, 14, 16672–16680.
- 7 Nguyen, N. T.; Kim, J.; Le, X. T.; Lee, W. T.; Lee, E. S.; Oh, K. T.; Choi, H.-G.; Youn, Y. S. Amplified Fenton-Based Oxidative Stress Utilizing Ultraviolet Upconversion Luminescence-Fueled Nanoreactors for Apoptosis-Strengthened Ferroptosis Anticancer Therapy. ACS Nano 2023, 17, 382–401.
- 8 Cakmak, Y.; Kolemen, S.; Duman, S.; Dede, Y.; Dolen, Y.; Kilic, B.; Kostereli, Z.; Yildirim, L. T.; Dogan, A. L.; Guc, D.; Akkaya, E. U. Designing Excited States: Theory-Guided Access to Efficient Photosensitizers for Photodynamic Action. Angew. Chem. Int. Ed. 2011, 50, 11937–11941.
- 9 Liang, H.; Liu, X. Y.; Tang, L. T.; Mahmood, Z.; Chen, Z. D.; Chen, G. W.; Ji, S. M.; Huo, Y. P. Heavy atom-free triplet photosensitizer based on thermally activated delayed fluorescence material for NIR-to-blue triplet-triplet annihilation upconversion. Chin. Chem. Lett. 2023, 34, 107515.
- 10 Baluschev, S.; Yakutkin, V.; Miteva, T.; Avlasevich, Y.; Chernov, S.; Aleshchenkov, S.; Nelles, G.; Cheprakov, A.; Yasuda, A.; Müllen, K.; Wegner, G. Blue-Green Up-Conversion: Noncoherent Excitation by NIR Light. Angew. Chem. Int. Ed. 2007, 46, 7693–7696.
- 11 Singh-Rachford, T. N.; Islangulov, R. R.; Castellano, F. N. Photochemical Upconversion Approach to Broad-Band Visible Light Generation. J. Phys. Chem. A 2008, 112, 3906–3910.
- 12 He, C.; Huang, R. L.; Wei, L. L.; He, Q. H.; Liu, J. B.; Chen, J.; Gao, G.; Yang, C.; Wu, W. H. Uncovering the mask of sensitizers to switch on the TTA-UC emission by supramolecular host-guest complexation. Chin. Chem. Lett. 2025, 36, 110103.
- 13 Kashino, T.; Hosoyamada, M.; Haruki, R.; Harada, N.; Yanai, N.; Kimizuka, N. Bulk Transparent Photon Upconverting Films by Dispersing High-Concentration Ionic Emitters in Epoxy Resins. ACS Appl. Mater. Interfaces 2021, 13, 13676–13683.
- 14 Raišys, S.; Adomėnienė, O.; Adomėnas, P.; Rudnick, A.; Köhler, A.; Kazlauskas, K. Triplet Exciton Diffusion and Quenching in Matrix-Free Solid Photon Upconversion Films. J. Phys. Chem. C 2021, 125, 3764–3775.
- 15 Turshatov, A.; Busko, D.; Kiseleva, N.; Grage, S. L.; Howard, I. A.; Richards, B. S. Room-Temperature High-Efficiency Solid-State Triplet–Triplet Annihilation Up-Conversion in Amorphous Poly(olefin sulfone)s. ACS Appl. Mater. Interfaces 2017, 9, 8280–8286.
- 16 Raišys, S.; Kazlauskas, K.; Juršėnas, S.; Simon, Y. C. The Role of Triplet Exciton Diffusion in Light-Upconverting Polymer Glasses. ACS Appl. Mater. Interfaces 2016, 8, 15732–15740.
- 17 Marsico, F.; Turshatov, A.; Peköz, R.; Avlasevich, Y.; Wagner, M.; Weber, K.; Donadio, D.; Landfester, K.; Baluschev, S.; Wurm, F. R. Hyperbranched Unsaturated Polyphosphates as a Protective Matrix for Long-Term Photon Upconversion in Air. J. Am. Chem. Soc. 2014, 136, 11057–11064.
- 18 Zhou, H. Q.; Lin, J. X.; Wan, S. G.; Lu, W. Photochemically deoxygenating gels for triplet–triplet annihilation photon-upconversion performed under air. PCCP 2022, 24, 29151–29158.
- 19 Enomoto, R.; Hoshi, M.; Oyama, H.; Agata, H.; Kurokawa, S.; Kuma, H.; Uekusa, H.; Murakami, Y. van der Waals solid solution crystals for highly efficient in-air photon upconversion under subsolar irradiance. Mater. Horiz. 2021, 8, 3449–3456.
- 20 Kamada, K.; Sakagami, Y.; Mizokuro, T.; Fujiwara, Y.; Kobayashi, K.; Narushima, K.; Hirata, S.; Vacha, M. Efficient triplet–triplet annihilation upconversion in binary crystalline solids fabricated via solution casting and operated in air. Mater. Horiz. 2017, 4, 83–87.
- 21 Wu, M. F.; Congreve, D. N.; Wilson, M. W. B.; Jean, J.; Geva, N.; Welborn, M.; Van Voorhis, T.; Bulović, V.; Bawendi, M. G.; Baldo, M. A. Solid-state infrared-to-visible upconversion sensitized by colloidal nanocrystals. Nat. Photonics 2016, 10, 31–34.
- 22 Ronchi, A.; Capitani, C.; Pinchetti, V.; Gariano, G.; Zaffalon, M. L.; Meinardi, F.; Brovelli, S.; Monguzzi, A. High Photon Upconversion Efficiency with Hybrid Triplet Sensitizers by Ultrafast Hole-Routing in Electronic-Doped Nanocrystals. Adv. Mater. 2020, 32, 2002953.
- 23 Wang, Y.; Wu, H.; Jones, L. O.; Mosquera, M. A.; Stern, C. L.; Schatz, G. C.; Stoddart, J. F. Color-Tunable Upconversion-Emission Switch Based on Cocrystal-to-Cocrystal Transformation. J. Am. Chem. Soc. 2023, 145, 1855–1865.
- 24 Gharaati, S.; Wang, C.; Förster, C.; Weigert, F.; Resch-Genger, U.; Heinze, K. Triplet–Triplet Annihilation Upconversion in a MOF with Acceptor-Filled Channels. Chem. Eur. J. 2020, 26, 1003–1007.
- 25 Meinardi, F.; Ballabio, M.; Yanai, N.; Kimizuka, N.; Bianchi, A.; Mauri, M.; Simonutti, R.; Ronchi, A.; Campione, M.; Monguzzi, A. Quasi- thresholdless Photon Upconversion in Metal–Organic Framework Nanocrystals. Nano Lett. 2019, 19, 2169–2177.
- 26 Mahato, P.; Monguzzi, A.; Yanai, N.; Yamada, T.; Kimizuka, N. Fast and long-range triplet exciton diffusion in metal–organic frameworks for photon upconversion at ultralow excitation power. Nat. Mater. 2015, 14, 924–930.
- 27 Bharmoria, P.; Hisamitsu, S.; Nagatomi, H.; Ogawa, T.; Morikawa, M.-a.; Yanai, N.; Kimizuka, N. Simple and Versatile Platform for Air- Tolerant Photon Upconverting Hydrogels by Biopolymer–Surfactant–Chromophore Co-assembly. J. Am. Chem. Soc. 2018, 140, 10848–10855.
- 28 Roy, I.; Goswami, S.; Young, R. M.; Schlesinger, I.; Mian, M. R.; Enciso, A. E.; Zhang, X.; Hornick, J. E.; Farha, O. K.; Wasielewski, M. R.; Hupp, J. T.; Stoddart, J. F. Photon Upconversion in a Glowing Metal–Organic Framework. J. Am. Chem. Soc. 2021, 143, 5053–5059.
- 29 Wei, L. L.; Fan, C. Y.; Rao, M.; Gao, F. R.; He, C.; Sun, Y. J.; Zhu, S. J.; He, Q. H.; Yang, C.; Wu, W. H. Triplet–triplet annihilation upconversion in LAPONITE®/PVP nanocomposites: absolute quantum yields of up to 23.8% in the solid state and application to anti-counterfeiting. Mater. Horiz. 2022, 9, 3048–3056.
- 30 Vadrucci, R.; Weder, C.; Simon, Y. C. Organogels for low-power light upconversion. Mater. Horiz. 2015, 2, 120–124.
- 31 Liu, X. C.; Fei, J. B.; Zhu, P. L.; Li, J. B. Facile Co-Assembly of a Dipeptide-Based Organogel toward Efficient Triplet–Triplet Annihilation Photonic Upconversion. Chem. Asian J. 2016, 11, 2700–2704.
- 32 Liu, X. Y.; He, Q.; Pan, J. G.; Liang, H.; Rehmat, N.; Gao, L.; Huo, Y. P.; Ji, S. M. Highly stable and air-resistant photonic upconversion organogels with self-healing and temperature responsiveness. J. Mater. Chem. C 2023, 11, 480–487.
- 33 Duan, P. F.; Yanai, N.; Nagatomi, H.; Kimizuka, N. Photon Upconversion in Supramolecular Gel Matrixes: Spontaneous Accumulation of Light-Harvesting Donor–Acceptor Arrays in Nanofibers and Acquired Air Stability. J. Am. Chem. Soc. 2015, 137, 1887–1894.
- 34 Sripathy, K.; MacQueen, R. W.; Peterson, J. R.; Cheng, Y. Y.; Dvořák, M.; McCamey, D. R.; Treat, N. D.; Stingelin, N.; Schmidt, T. W. Highly efficient photochemical upconversion in a quasi-solid organogel. J. Mater. Chem. C 2015, 3, 616–622.
- 35 Chai, Q.; Wei, J.; Bai, B. L.; Wang, H. T.; Li, M. A Photo-Responsive Organogel Based on Pyrene-Substituted Acylhydrazone Derivative. Chin. J. Chem. 2017, 35, 1829–1834.
- 36 Wang, Y. X.; Yuan, Y.; Zhang, S. P.; Chen, L.; Chen, Y. L. Cholesterol Decorated Diazocine Gelator with Photo- and Thermo-Responsive Properties for Smart Window. Chin. J. Chem. 2024, 42, 3278–3282.
- 37 Yang, H. K.; Zhang, C.; He, X. N.; Wang, P. Y. Effects of alkyl chain lengths on 12-hydroxystearic acid derivatives based supramolecular organogels. Colloids Surf. Physicochem. Eng. Aspects 2021, 616, 126319.
- 38 Wang, C. S.; Li, Z. Y.; Wang, X. H.; Wei, W.; Chen, S. D.; Sui, Z. T. Gelation mechanism and microstructure of organogels formed with l-Valine dihydrazide derivatives. Colloids Surf. Physicochem. Eng. Aspects 2011, 384, 490–495.
- 39 Lv, Y. J.; Xiao, C.; Ma, J. Y.; Zhou, D. Y.; Wu, W. H.; Yang, C. Solvent and guest-binding-controlled chiroptical inversion of molecular devices based on pseudo[1]catenane-type pillar[5]arene derivatives. Chin. Chem. Lett. 2024, 35, 108757.
- 40 Yang, D.; Han, J. L.; Sang, Y. T.; Zhao, T. H.; Liu, M. H.; Duan, P. F. Steering Triplet–Triplet Annihilation Upconversion through Enantioselective Self-Assembly in a Supramolecular Gel. J. Am. Chem. Soc. 2021, 143, 13259–13265.
- 41 Fan, C. Y.; Wu, W. H.; Chruma, J. J.; Zhao, J. Z.; Yang, C. Enhanced Triplet–Triplet Energy Transfer and Upconversion Fluorescence through Host–Guest Complexation. J. Am. Chem. Soc. 2016, 138, 15405–15412.
- 42 Fan, C. Y.; Wei, L. L.; Niu, T.; Rao, M.; Cheng, G.; Chruma, J. J.; Wu, W. H.; Yang, C. Efficient Triplet–Triplet Annihilation Upconversion with an Anti-Stokes Shift of 1.08 eV Achieved by Chemically Tuning Sensitizers. J. Am. Chem. Soc. 2019, 141, 15070–15077.
- 43 Lai, H. X.; Zhao, T.; Deng, Y. R.; Fan, C. Y.; Wu, W. H.; Yang, C. Assembly-enhanced triplet-triplet annihilation upconversion in the aggregation formed by Schiff-base Pt(II) complex grafting-permethyl-β-CD and 9,10-diphenylanthracence dimer. Chin. Chem. Lett. 2019, 30, 1979–1983.
- 44 He, Q. H.; Wei, L. L.; He, C.; Yang, C.; Wu, W. H., Supramolecular Annihilator with DPA Parallelly Arranged by Multiple Hydrogen-Bonding Interactions for Enhanced Triplet–Triplet Annihilation Upconversion. Molecules 2024, 29, 2203.
- 45 Mallia, V. A.; George, M.; Blair, D. L.; Weiss, R. G. Robust Organogels from Nitrogen-Containing Derivatives of (R)-12-Hydroxystearic Acid as Gelators: Comparisons with Gels from Stearic Acid Derivatives. Langmuir 2009, 25, 8615–8625.
- 46 Zheng, M.; Li, Y. M.; Wei, Y. X.; Chen, L.; Liu, S. L.; Zhou, X. G. Highly Efficient Triplet–Triplet Annihilation Upconversion with a Thermally Activated Delayed Fluorescence Molecule as Triplet Photosensitizer. J. Phys. Chem. C 2023, 127, 2846–2854.
- 47 Wei, Y. X.; Wang, Y.; Zhou, Q. H.; Zhang, S.; Zhang, B.; Zhou, X. G.; Liu, S. L. Solvent effects on triplet–triplet annihilation upconversion kinetics of perylene with a Bodipy-phenyl-C60 photosensitizer. PCCP 2020, 22, 26372–26382.
- 48 Peng, C.; Liang, W. T.; Ji, J. C.; Fan, C. Y.; Kanagaraj, K.; Wu, W. H.; Cheng, G.; Su, D.; Zhong, Z. H.; Yang, C. Pyrene-tiaraed pillar[5]arene: Strong intramolecular excimer emission applicable for photo-writing. Chin. Chem. Lett. 2021, 32, 345–348.
- 49 Liu, X.; Fei, J.; Zhu, P.; Li, J. Facile Co-Assembly of a Dipeptide-Based Organogel toward Efficient Triplet–Triplet Annihilation Photonic Upconversion. Chem. Asian J. 2016, 11, 2700–2704.
- 50 Monguzzi, A.; Mezyk, J.; Scotognella, F.; Tubino, R.; Meinardi, F. Upconversion-induced fluorescence in multicomponent systems: Steady-state excitation power threshold. Phys. Rev. B 2008, 78, 195112.
- 51 Durandin, N. A.; Isokuortti, J.; Efimov, A.; Vuorimaa-Laukkanen, E.; Tkachenko, N. V.; Laaksonen, T. Critical Sensitizer Quality Attributes for Efficient Triplet–Triplet Annihilation Upconversion with Low Power Density Thresholds. J. Phys. Chem. C 2019, 123, 22865–22872.
- 52 Haefele, A.; Blumhoff, J.; Khnayzer, R. S.; Castellano, F. N. Getting to the (Square) Root of the Problem: How to Make Noncoherent Pumped Upconversion Linear. J. Phys. Chem. Lett. 2012, 3, 299–303.
- 53 Duan, P. F.; Asthana, D.; Nakashima, T.; Kawai, T.; Yanai, N.; Kimizuka, N. All-or-none switching of photon upconversion in self-assembled organogel systems. Faraday Discuss. 2017, 196, 305–316.
- 54 Wei, L. L.; Gao, F. R.; He, C.; He, Q. H.; Jin, P. Y.; Rong, Y. Q.; Zhao, T.; Yang, C.; Wu, W. H. A new sensitization strategy for achieving organic RTP in aqueous solution: tunable RTP and UC emission in supramolecular TTA-UC systems. Sci. China Chem. 2023, 66, 3546–3554.
- 55 Sun, Y. J.; Wei, L. L.; Zhu, S. J.; Jin, P. Y.; He, C.; He, Q. H.; Yang, C.; Wu, W. H. Stimuli-responsive triplet-triplet annihilation upconversion with guanidyl functionalized annihilators for enhanced ratiometric sensing of trace water in MeOH. Sens. Actuators B: Chem. 2023, 387, 133764.
- 56 Xu, W.; Liang, W. T.; Wu, W. H.; Fan, C. Y.; Rao, M.; Su, D.; Zhong, Z. H.; Yang, C. Supramolecular Assembly-Improved Triplet–Triplet Annihilation Upconversion in Aqueous Solution. Chem. Eur. J. 2018, 24, 16677–16685.




