A Self-immobilizing PET Tracer for Imaging of H2O2 Level in the Brain
Ke Li
NHC Key Laboratory of Nuclear Medicine, Jiangsu Key Laboratory of Molecular Nuclear Medicine, Jiangsu Institute of Nuclear Medicine, Wuxi, Jiangsu, 214063 China
Search for more papers by this authorShiliang Zhu
NHC Key Laboratory of Nuclear Medicine, Jiangsu Key Laboratory of Molecular Nuclear Medicine, Jiangsu Institute of Nuclear Medicine, Wuxi, Jiangsu, 214063 China
Search for more papers by this authorYing Peng
NHC Key Laboratory of Nuclear Medicine, Jiangsu Key Laboratory of Molecular Nuclear Medicine, Jiangsu Institute of Nuclear Medicine, Wuxi, Jiangsu, 214063 China
Search for more papers by this authorCorresponding Author
Ling Qiu
NHC Key Laboratory of Nuclear Medicine, Jiangsu Key Laboratory of Molecular Nuclear Medicine, Jiangsu Institute of Nuclear Medicine, Wuxi, Jiangsu, 214063 China
E-mail: [email protected]; [email protected]Search for more papers by this authorCorresponding Author
Jianguo Lin
NHC Key Laboratory of Nuclear Medicine, Jiangsu Key Laboratory of Molecular Nuclear Medicine, Jiangsu Institute of Nuclear Medicine, Wuxi, Jiangsu, 214063 China
E-mail: [email protected]; [email protected]Search for more papers by this authorKe Li
NHC Key Laboratory of Nuclear Medicine, Jiangsu Key Laboratory of Molecular Nuclear Medicine, Jiangsu Institute of Nuclear Medicine, Wuxi, Jiangsu, 214063 China
Search for more papers by this authorShiliang Zhu
NHC Key Laboratory of Nuclear Medicine, Jiangsu Key Laboratory of Molecular Nuclear Medicine, Jiangsu Institute of Nuclear Medicine, Wuxi, Jiangsu, 214063 China
Search for more papers by this authorYing Peng
NHC Key Laboratory of Nuclear Medicine, Jiangsu Key Laboratory of Molecular Nuclear Medicine, Jiangsu Institute of Nuclear Medicine, Wuxi, Jiangsu, 214063 China
Search for more papers by this authorCorresponding Author
Ling Qiu
NHC Key Laboratory of Nuclear Medicine, Jiangsu Key Laboratory of Molecular Nuclear Medicine, Jiangsu Institute of Nuclear Medicine, Wuxi, Jiangsu, 214063 China
E-mail: [email protected]; [email protected]Search for more papers by this authorCorresponding Author
Jianguo Lin
NHC Key Laboratory of Nuclear Medicine, Jiangsu Key Laboratory of Molecular Nuclear Medicine, Jiangsu Institute of Nuclear Medicine, Wuxi, Jiangsu, 214063 China
E-mail: [email protected]; [email protected]Search for more papers by this authorComprehensive Summary
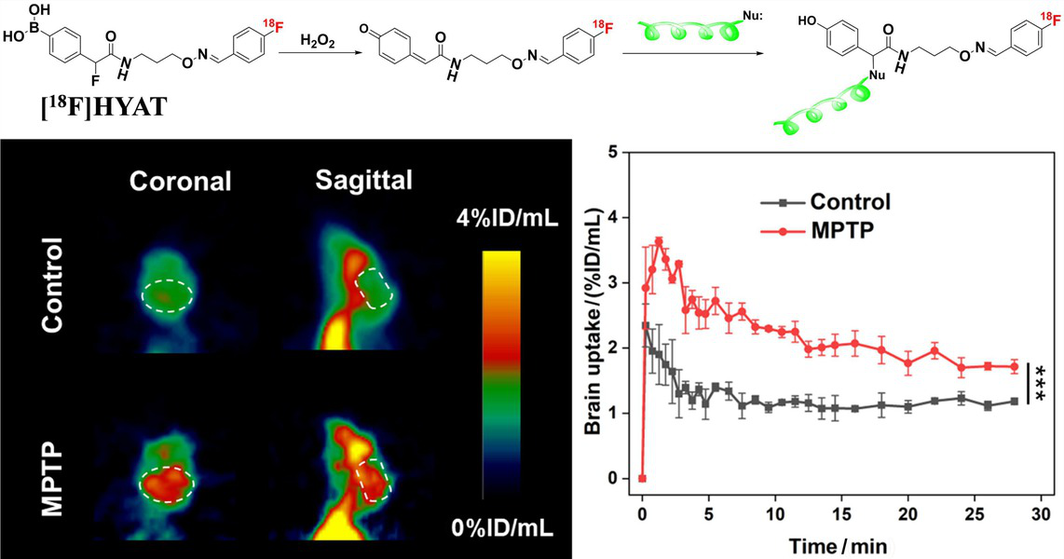
Reactive oxygen species (ROS) are involved in the onset and development of neurodegenerative diseases, such as Alzheimer's disease (AD) and Parkinson's disease (PD). Hydrogen peroxide (H2O2), a key type of ROS, is overexpressed in the early stages of AD and PD and is involved in the disease progression. Assessing H2O2 levels in the brain is considered to be a valuable tool for detecting neurodegenerative diseases and exploring their pathogenesis. In this study, we developed two self-immobilizing PET tracers ([18F]HYAS and [18F]HYAT) based on a quinone methide (QM) scaffold for non-invasive imaging of H2O2 in the brain. Both tracers can respond to H2O2 by forming a QM intermediate, which rapidly reacts with nucleophiles. [18F]HYAT, with proper physicochemical properties, is able of crossing the blood-brain barrier. Increased uptake of [18F]HYAT was observed in the brains of mice treated with 1-methyl-4-phenyl- l,2,3,6-tetrahydropyridine (MPTP), indicating that [18F]HYAT is a useful tracer for PET imaging of H2O2 in the brain.
Supporting Information
| Filename | Description |
|---|---|
| cjoc202401286-sup-0001-supinfo.pdfPDF document, 3.1 MB |
Appendix S1: Supporting Information |
Please note: The publisher is not responsible for the content or functionality of any supporting information supplied by the authors. Any queries (other than missing content) should be directed to the corresponding author for the article.
References
- 1 Wang, X.; Wang, C.; Chan, H.-N.; Ashok, I.; Krishnamoorthi, S. K.; Li, M.; Li, H.-W.; Wong, M. S. Amyloid-β oligomer targeted theranostic probes for in vivo NIR imaging and inhibition of self-aggregation and amyloid-β induced ROS generation. Talanta 2021, 224, 121830.
- 2 Barnham, K. J.; Masters, C. L.; Bush, A. I. Neurodegenerative diseases and oxidative stress. Nat. Rev. Drug Discov. 2004, 3, 205–214.
- 3 Kim, G. H.; Kim, J. E.; Rhie, S. J.; Yoon, S. The Role of Oxidative Stress in Neurodegenerative Diseases. Exp. Neurobiol. 2015, 24, 325–340.
- 4 Abdelhamid, R. F.; Nagano, S. Crosstalk between Oxidative Stress and Aging in Neurodegeneration Disorders. Cells 2023, 12, 753.
- 5 Briyal, S.; Ranjan, A. K.; Gulati, A. Oxidative stress: A target to treat Alzheimer's disease and stroke. Neurochem. Int. 2023, 165, 105509.
- 6 Sies, H.; Jones, D. P. Reactive oxygen species (ROS) as pleiotropic physiological signalling agents. Nat. Rev. Mol. Cell Biol. 2020, 21, 363–383.
- 7 Tabner, B. J.; El-Agnaf, O. M. A.; Turnbull, S.; German, M. J.; Paleologou, K. E.; Hayashi, Y.; Cooper, L. J.; Fullwood, N. J.; Allsop, D. Hydrogen Peroxide Is Generated during the Very Early Stages of Aggregation of the Amyloid Peptides Implicated in Alzheimer Disease and Familial British Dementia. J. Biol. Chem. 2005, 280, 35789–35792.
- 8 Tabner, B. J.; Turnbull, S.; Fullwood, N. J.; German, M.; Allsop, D. The production of hydrogen peroxide during early-stage protein aggregation: a common pathological mechanism in different neurodegenerative diseases? Biochem. Soc. Trans. 2005, 33, 548–550.
- 9 Lee, Y. M.; He, W.; Liou, Y.-C. The redox language in neurodegenerative diseases: oxidative post-translational modifications by hydrogen peroxide. Cell Death Dis. 2021, 12, 58.
- 10 Umeno, A.; Biju, V.; Yoshida, Y. In vivo ROS production and use of oxidative stress-derived biomarkers to detect the onset of diseases such as Alzheimer's disease, Parkinson's disease, and diabetes. Free Radical Res. 2017, 51, 413–427.
- 11 Zielonka, J.; Kalyanaraman, B. Small-molecule luminescent probes for the detection of cellular oxidizing and nitrating species. Free Radical Biol. Med. 2018, 128, 3–22.
- 12 Espinoza, E. M.; Røise, J. J.; Li, I.-C.; Das, R.; Murthy, N. Advances in imaging reactive oxygen species. J. Nucl. Med. 2021, 62, 457–461.
- 13 Huang, L.; Li, Z.; Zhang, X. Radiotracers for Nuclear Imaging of Reactive Oxygen Species: Advances Made So Far. Bioconjugate Chem. 2022, 33, 749–766.
- 14 Mei, Y.; Li, Z.; Rong, K.; Hai, Z.; Tang, W.; Song, Q.-H. A BODIPY-based fluorescent probe for simultaneous detection of H2O2 and viscosity during the pyroptosis process. Chem. Commun. 2023, 59, 12775–12778.
- 15 Fan, L.; Zan, Q.; Wang, X.; Wang, S.; Zhang, Y.; Dong, W.; Shuang, S.; Dong, C. A Mitochondria-Specific Orange/Near-Infrared-Emissive Fluorescent Probe for Dual-Imaging of Viscosity and HO in Inflammation and Tumor Models. Chin. J. Chem. 2021, 39, 1303–1309.
- 16 Xu, L.; Sun, L.; Zeng, F.; Wu, S. Near-Infrared Fluorescent Nanoprobe for Detecting Hydrogen Peroxide in Inflammation and Ischemic Kidney Injury. Chin. J. Chem. 2020, 38, 1304–1310.
- 17 Carroll, V.; Michel, B. W.; Blecha, J.; VanBrocklin, H.; Keshari, K.; Wilson, D.; Chang, C. J. A Boronate-Caged [18F]FLT Probe for Hydrogen Peroxide Detection Using Positron Emission Tomography. J. Am. Chem. Soc. 2014, 136, 14742–14745.
- 18 Yang, J.; Zhu, B.; Ran, C. The Application of Bio-orthogonality for In Vivo Animal Imaging. Chem. Biomed. Imaging 2023, 1, 434–447.
- 19 Shabsigh, M.; Solomon, L. A. Peptide PET Imaging: A Review of Recent Developments and a Look at the Future of Radiometal-Labeled Peptides in Medicine. Chem. Biomed. Imaging 2024, 2, 615–630.
- 20 Huang, W.; Yang, Y.; Ye, D. Recent Advances in Activatable Probes for Molecular Imaging by Stimuli-Controlled Disassembly. Chin. J. Chem. 2023, 41, 2382–2399.
- 21 Geng, Y.; Wang, Z.; Zhou, J.; Zhu, M.; Liu, J.; James, T. D. Recent progress in the development of fluorescent probes for imaging pathological oxidative stress. Chem. Soc. Rev. 2023, 52, 3873–3926.
- 22 Lin, S.; Ye, C.; Lin, Z.; Huang, L.; Li, D. Recent progress of near-infrared fluorescent probes in the determination of reactive oxygen species for disease diagnosis. Talanta 2024, 268, 125264.
- 23 Abe, A.; Kamiya, M. A versatile toolbox for investigating biological processes based on quinone methide chemistry: From self-immolative linkers to self-immobilizing agents. Bioorg. Med. Chem. 2021, 44, 116281.
- 24 Egami, H.; Nakagawa, S.; Katsura, Y.; Kanazawa, M.; Nishiyama, S.; Sakai, T.; Arano, Y.; Tsukada, H.; Inoue, O.; Todoroki, K.; Hamashima, Y. (18)F-Labeled dihydromethidine: positron emission tomography radiotracer for imaging of reactive oxygen species in intact brain. Org. Biomol. Chem. 2020, 18, 2387–2391.
- 25 Chu, W.; Chepetan, A.; Zhou, D.; Shoghi, K. I.; Xu, J.; Dugan, L. L.; Gropler, R. J.; Mintun, M. A.; Mach, R. H. Development of a PET radiotracer for non-invasive imaging of the reactive oxygen species, superoxide, in vivo. Org. Biomol. Chem. 2014, 12, 4421–4431.
- 26 Hou, C.; Hsieh, C. J.; Li, S.; Lee, H.; Graham, T. J.; Xu, K.; Weng, C. C.; Doot, R. K.; Chu, W.; Chakraborty, S. K.; Dugan, L. L.; Mintun, M. A.; Mach, R. H. Development of a Positron Emission Tomography Radiotracer for Imaging Elevated Levels of Superoxide in Neuroinflammation. ACS Chem. Neurosci. 2018, 9, 578–586.
- 27 Yan, J.; Liu, H.; Wu, Y.; Niu, B.; Deng, X.; Zhang, L.; Dang, Q.; Wang, Y.; Lu, X.; Zhang, B.; Sun, W. Recent progress of self-immobilizing and self-precipitating molecular fluorescent probes for higher-spatial- resolution imaging. Biomaterials 2023, 301, 122281.
- 28 Zhu, R.; Wang, S.; Xue, Z.; Han, J.; Han, S. Senescence-associated sialidase revealed by an activatable fluorescence-on labeling probe. Chem. Commun. 2018, 54, 11566–11569.
- 29 Chen, J. W.; Wu, T. C.; Liang, W.; Ciou, J.-J.; Lai, C.-H. Boronates as hydrogen peroxide–reactive warheads in the design of detection probes, prodrugs, and nanomedicines used in tumors and other diseases. Drug Delivery Transl. Res. 2023, 13, 1305–1321.
- 30 Yang, G.; Zhu, T.; Wang, D.; Liu, Z.; Zhang, R.; Han, G.; Tian, X.; Liu, B.; Han, M.-y.; Zhang, Z. Revealing the signaling regulation of hydrogen peroxide to cell pyroptosis using a ratiometric fluorescent probe in living cells. Chem. Commun. 2021, 57, 6628–6631.
- 31 Yang, J.; Yang, J.; Liang, S. H.; Xu, Y.; Moore, A.; Ran, C. Imaging hydrogen peroxide in Alzheimer's disease via cascade signal amplification. Sci. Rep. 2016, 6, 35613.
- 32 Gnaim, S.; Shabat, D. Quinone-methide species, a gateway to functional molecular systems: from self-immolative dendrimers to long- wavelength fluorescent dyes. Acc. Chem. Res. 2014, 47, 2970–2984.
- 33 Zhong, D.; Xiong, S.; Zhang, Y.; Cui, M.; Liu, L.; Xu, Y.; Wang, P.; Zhang, W. H2O2-activated NIR fluorescent probe with tumor targeting for cell imaging and fluorescent-guided surgery. Sens. Actuators B: Chem. 2024, 418, 136249.
- 34 Van de Bittner, G. C.; Dubikovskaya, E. A.; Bertozzi, C. R.; Chang, C. J. In vivo imaging of hydrogen peroxide production in a murine tumor model with a chemoselective bioluminescent reporter. Proc. Natl. Acad. Sci. 2010, 107, 21316–21321.
- 35 Xiong, B.; Wang, Y.; Chen, Y.; Xing, S.; Liao, Q.; Chen, Y.; Li, Q.; Li, W.; Sun, H. Strategies for Structural Modification of Small Molecules to Improve Blood–Brain Barrier Penetration: A Recent Perspective. J. Med. Chem. 2021, 64, 13152–13173.
- 36 Jackson-Lewis, V.; Przedborski, S. Protocol for the MPTP mouse model of Parkinson's disease. Nat. Protoc. 2007, 2, 141–151.
- 37 Kurth, J. H.; Kurth, M. C.; Poduslo, S. E.; Schwankhaus, J. D. Association of a monoamine oxidase B allele with Parkinson's disease. Ann. Neurol. 1993, 33, 368–372.
- 38 Sedelis, M.; Hofele, K.; Auburger, G. W.; Morgan, S.; Huston, J. P.; Schwarting, R. K. W. MPTP Susceptibility in the Mouse: Behavioral, Neurochemical, and Histological Analysis of Gender and Strain Differences. Behav. Genet. 2000, 30, 171–182.
- 39 Adams, J. D.; Odunze, I. N. Biochemical mechanisms of 1-methyl-4- phenyl-1,2,3,6-tetrahydropyridine toxicity: Could oxidative stressbe involved in the brain? Biochem. Pharmacol. 1991, 41, 1099–1105.
- 40 Chen, X.; Wang, D.; Zhang, L.; Yao, H.; Zhu, H.; Zhao, N.; Peng, X.; Yang, K. Neuroprotective Effect of Low-Intensity Pulsed Ultrasound on the Mouse MPTP/MPP+ Model of Dopaminergic Neuron Injury. Ultrasound Med. Biol. 2021, 47, 2321–2330.
- 41 Johannessen, J. N.; Adams, J. D.; Schuller, H. M.; Bacon, J. P.; Markey, S. P. 1-Methyl-4-phenylpyridine (MPP+) induces oxidative stress in the rodent. Life Sci. 1986, 38, 743–749.
- 42 Li, L.; Shi, L.; Liu, H.; Luo, Q.; Huang, C.; Liu, W.; Chen, X.; Zeng, W.; Chen, Z. Changes in blood anti-oxidation enzyme levels in MPTP- treated monkeys. Neurosci. Lett. 2017, 649, 93–99.
- 43 Wen, L.; Zhang, Q. S.; Heng, Y.; Chen, Y.; Wang, S.; Yuan, Y. H.; Chen, N. H. NLRP3 inflammasome activation in the thymus of MPTP-induced Parkinsonian mouse model. Toxicol. Lett. 2018, 288, 1–8.
- 44 Di Monte, D.; Ekström, G.; Shinka, T.; Smith, M. T.; Trevor, A. J.; Castagnoli Jr, N. Role of 1-methyl-4-phenylpyridinium ion formation and accumulation in 1-methyl-4-phenyl-1, 2, 3, 6-tetrahydropyridine toxicity to isolated hepatocytes. Chem. Biol. Interact. 1987, 62, 105–116.
- 45 Qiao, C.; Zhang, Q.; Jiang, Q.; Zhang, T.; Chen, M.; Fan, Y.; Ding, J.; Lu, M.; Hu, G. Inhibition of the hepatic Nlrp3 protects dopaminergic neurons via attenuating systemic inflammation in a MPTP/p mouse model of Parkinson's disease. J. Neuroinflammation 2018, 15, 193.
- 46 Richarz, R.; Krapf, P.; Zarrad, F.; Urusova, E. A.; Neumaier, B.; Zlatopolskiy, B. D. Neither azeotropic drying, nor base nor other additives: a minimalist approach to (18)F-labeling. Org. Biomol. Chem. 2014, 12, 8094–8099.




