Heterogeneous Organic Polymers Embedded with Chiral Bisoxazoline Ligands for Photoinduced Cu-Catalyzed Asymmetric Cyanation
Ren-Jie Yu
Engineering Research Center of Photoenergy Utilization for Pollution Control and Carbon Reduction, Ministry of Education, College of Chemistry, Central China Normal University, 152 Luoyu Road, Wuhan, Hubei, 430079 China
‡ These authors contributed equally to this work.
Search for more papers by this authorRui-Rui Zhao
Engineering Research Center of Photoenergy Utilization for Pollution Control and Carbon Reduction, Ministry of Education, College of Chemistry, Central China Normal University, 152 Luoyu Road, Wuhan, Hubei, 430079 China
‡ These authors contributed equally to this work.
Search for more papers by this authorFang Hu
State Key Laboratory of Chemical Oncogenomics, Key Laboratory of Chemical Biology, Tsinghua Shenzhen International Graduate School, Tsinghua University, Shenzhen, Guangdong, 518055 China
Search for more papers by this authorCorresponding Author
Zhen Su
Engineering Research Center of Photoenergy Utilization for Pollution Control and Carbon Reduction, Ministry of Education, College of Chemistry, Central China Normal University, 152 Luoyu Road, Wuhan, Hubei, 430079 China
Wuhan Institute of Photochemistry and Technology, Wuhan, Hubei, 430082 China
E-mail [email protected]; [email protected]; [email protected]Search for more papers by this authorCorresponding Author
Ke Gao
Engineering Research Center of Photoenergy Utilization for Pollution Control and Carbon Reduction, Ministry of Education, College of Chemistry, Central China Normal University, 152 Luoyu Road, Wuhan, Hubei, 430079 China
Wuhan Institute of Photochemistry and Technology, Wuhan, Hubei, 430082 China
E-mail [email protected]; [email protected]; [email protected]Search for more papers by this authorCorresponding Author
Liang-Qiu Lu
Engineering Research Center of Photoenergy Utilization for Pollution Control and Carbon Reduction, Ministry of Education, College of Chemistry, Central China Normal University, 152 Luoyu Road, Wuhan, Hubei, 430079 China
Wuhan Institute of Photochemistry and Technology, Wuhan, Hubei, 430082 China
State Key Laboratory for Oxo Synthesis and Selective Oxidation, Lanzhou Institute of Chemical Physics (LICP), Chinese Academy of Sciences, Lanzhou, Gansu, 730000 China
School of Chemistry and Chemical Engineering, Henan Normal University, Xinxiang, Henan, 453007 China
E-mail [email protected]; [email protected]; [email protected]Search for more papers by this authorWen-Jing Xiao
Engineering Research Center of Photoenergy Utilization for Pollution Control and Carbon Reduction, Ministry of Education, College of Chemistry, Central China Normal University, 152 Luoyu Road, Wuhan, Hubei, 430079 China
Wuhan Institute of Photochemistry and Technology, Wuhan, Hubei, 430082 China
Search for more papers by this authorRen-Jie Yu
Engineering Research Center of Photoenergy Utilization for Pollution Control and Carbon Reduction, Ministry of Education, College of Chemistry, Central China Normal University, 152 Luoyu Road, Wuhan, Hubei, 430079 China
‡ These authors contributed equally to this work.
Search for more papers by this authorRui-Rui Zhao
Engineering Research Center of Photoenergy Utilization for Pollution Control and Carbon Reduction, Ministry of Education, College of Chemistry, Central China Normal University, 152 Luoyu Road, Wuhan, Hubei, 430079 China
‡ These authors contributed equally to this work.
Search for more papers by this authorFang Hu
State Key Laboratory of Chemical Oncogenomics, Key Laboratory of Chemical Biology, Tsinghua Shenzhen International Graduate School, Tsinghua University, Shenzhen, Guangdong, 518055 China
Search for more papers by this authorCorresponding Author
Zhen Su
Engineering Research Center of Photoenergy Utilization for Pollution Control and Carbon Reduction, Ministry of Education, College of Chemistry, Central China Normal University, 152 Luoyu Road, Wuhan, Hubei, 430079 China
Wuhan Institute of Photochemistry and Technology, Wuhan, Hubei, 430082 China
E-mail [email protected]; [email protected]; [email protected]Search for more papers by this authorCorresponding Author
Ke Gao
Engineering Research Center of Photoenergy Utilization for Pollution Control and Carbon Reduction, Ministry of Education, College of Chemistry, Central China Normal University, 152 Luoyu Road, Wuhan, Hubei, 430079 China
Wuhan Institute of Photochemistry and Technology, Wuhan, Hubei, 430082 China
E-mail [email protected]; [email protected]; [email protected]Search for more papers by this authorCorresponding Author
Liang-Qiu Lu
Engineering Research Center of Photoenergy Utilization for Pollution Control and Carbon Reduction, Ministry of Education, College of Chemistry, Central China Normal University, 152 Luoyu Road, Wuhan, Hubei, 430079 China
Wuhan Institute of Photochemistry and Technology, Wuhan, Hubei, 430082 China
State Key Laboratory for Oxo Synthesis and Selective Oxidation, Lanzhou Institute of Chemical Physics (LICP), Chinese Academy of Sciences, Lanzhou, Gansu, 730000 China
School of Chemistry and Chemical Engineering, Henan Normal University, Xinxiang, Henan, 453007 China
E-mail [email protected]; [email protected]; [email protected]Search for more papers by this authorWen-Jing Xiao
Engineering Research Center of Photoenergy Utilization for Pollution Control and Carbon Reduction, Ministry of Education, College of Chemistry, Central China Normal University, 152 Luoyu Road, Wuhan, Hubei, 430079 China
Wuhan Institute of Photochemistry and Technology, Wuhan, Hubei, 430082 China
Search for more papers by this authorComprehensive Summary
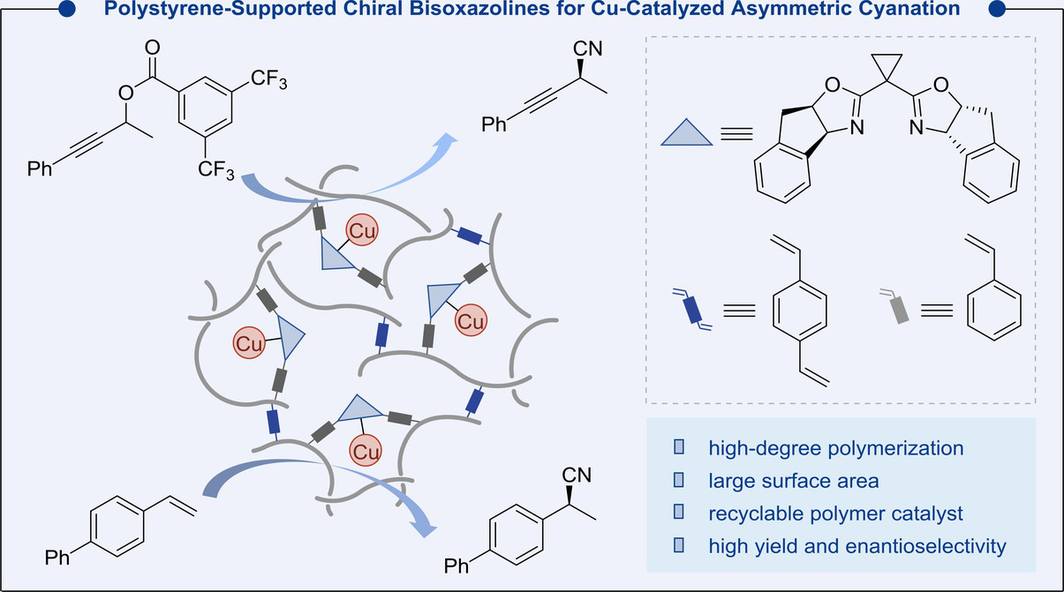
Chiral bisoxazoline (box) ligands with indene groups at C4 and C5 are highly potent in asymmetric catalysis, but face challenges in terms of cost and recyclability. To address this, we have designed polystyrene-supported box ligands by modifying the indene moiety instead of the traditional methylene bridge. This design preserves the necessary steric environment for copper coordination, enabling high efficiency and excellent enantioselectivity as examined in photoinduced asymmetric cyanation reactions. The resulting copper complexes are robust and recyclable, maintaining performance over five cycles. This approach provides a sustainable and practical solution for asymmetric catalysis with chiral box ligands.
Supporting Information
| Filename | Description |
|---|---|
| cjoc202500106-sup-0001-supinfo.pdfPDF document, 2 MB |
Appendix S1: Supporting Information |
Please note: The publisher is not responsible for the content or functionality of any supporting information supplied by the authors. Any queries (other than missing content) should be directed to the corresponding author for the article.
References
- 1 Eriksson, T.; Bjöurkman, S.; Roth, B.; Fyge, Å.; Höuglund, P. Stereospecific determination, chiral inversion in vitro and pharmacokinetics in humans of the enantiomers of thalidomide. Chirality 1995, 7, 44–52.
- 2 Mehvar, R.; Brocks, D. R.; Vakily, M. Impact of stereoselectivity on the pharmacokinetics and pharmacodynamics of antiarrhythmic drugs. Clin. Pharmacokinet. 2002, 41, 533–558.
- 3(a) Huo, H.; Meggersa, E. Cooperative photoredox and asymmetric catalysis. Chimia 2016, 70, 186–186; (b) Imamoto, T. P-stereogenic phosphorus ligands in asymmetric catalysis. Chem. Rev. 2024, 124, 8657–8739; (c) Wakchaure, V. N.; DeSnoo, W.; Laconsay, C. J.; Leutzsch, M.; Tsuji, N.; Tantillo, D. J.; List, B. Catalytic asymmetric cationic shifts of aliphatic hydrocarbons. Nature 2024, 625, 287–292; (d) Zhao, H. T.; Lin, J. N.; Shu, W. Visible-light mediated nickel-catalyzed asymmetric difunctionalizations of alkenes. Chem. Eur. J. 2024, e202402712; (e) Heitbaum, M.; Glorius, F.; Escher, I. Asymmetric heterogeneous catalysis. Angew. Chem. Int. Ed. 2006, 45, 4732–4762.
- 4(a) Hargaden, G. C.; Guiry, P. J. Recent applications of oxazoline- containing ligands in asymmetric catalysis. Chem. Rev. 2009, 109, 2505–2550; (b) Desimoni, G.; Faita, G.; Jørgensen, K. A. C2-symmetric chiral bis(oxazolines) ligands in asymmetric catalysis. Chem. Rev. 2006, 106, 3561–3651.
- 5 Yang, B. Y.; Chen, X. M.; Deng, G. J.; Zhang, Y. L.; Fan, Q. H. Chiral dendritic bis(oxazolines) copper(II) complexes as lewis acid catalysts for enantioselective aldol reactions in aqueous media. Tetrahedron Lett. 2003, 44, 3535–3538.
- 6
Atodiresei, I.; Schiffers, I.; Bolm, C. Asymmetric synthesis of chiral bis(oxazolines) and their use as ligands in metal catalysis. Tetrahedron: Asymmetry 2006, 17, 620633.
10.1016/j.tetasy.2005.12.036 Google Scholar
- 7 Gathergood, N.; Zhuang, W.; Jørgensen, K. A. Catalytic enantioselective Friedel-Crafts reactions of aromatic compounds with glyoxylate: a simple procedure for the synthesis of optically active aromatic mandelic acid esters. J. Am. Chem. Soc. 2000, 122, 12517–12522.
- 8 Fraile, J. M.; García, J. I.; Mayoral, J. A. Recent advances in the immobilization of chiral catalysts containing bis(oxazolines) and related ligands. Coord. Chem. Rev. 2008, 252, 624–646.
- 9 Glos, M.; Reiser, O. Aza-bis (oxazolines): New chiral ligands for asymmetric catalysis. Org. Lett. 2000, 2, 2045–2048.
- 10 Burguete, M. I.; Fraile, J. M.; García, J. I.; García-Verdugo, E.; Herrerías, C. I.; Luis, S. V.; Mayoral, J. A. Bis(oxazolines) copper complexes covalently bonded to insoluble support as catalysts in cyclopropanation reactions. J. Org. Chem. 2001, 66, 8893–8901.
- 11 Clarke, R. J.; Shannon, I. J. Mesopore immobilised copper bis(oxazolines) complexes for enantioselective catalysis. Chem. Commun. 2001, 19, 1936–1937.
- 12 Annunziata, R.; Benaglia, M.; Cinquini, M.; Cozzi, F.; Pozzi, G. Synthesis of perfluoroalkyl-substituted bis(oxazolines) as ligands for catalytic enantioselective reactions. Eur. J. Org. Chem. 2003, 1191–1197.
- 13 Bayardon, J.; Holczknecht, O.; Pozzi, G.; Sinou, D. Asymmetric cyclopropanation catalyzed by fluorous bis(oxazolines)-copper complexes. Tetrahedron: Asymmetry 2006, 17, 1568–1572.
- 14(a) Das, S.; Heasman, P.; Ben, T.; Qiu, S. Porous organic materials: strategic design and structure-function correlation. Chem. Rev. 2017, 117, 1515–1563; (b) Zhang, Y.; Riduan, S. N. Functional porous organic polymers for heterogeneous catalysis. Chem. Soc. Rev. 2012, 41, 2083–2094; (c) Wang, W.; Zhou, M.; Yuan, D. Carbon dioxide capture in amorphous porous organic polymers. J. Mater. Chem. A 2017, 5, 1334–1347; (d) Chen, D.; Liu, C.; Tang, J.; Luo, L.; Yu, G. Fluorescent porous organic polymers. Polym. Chem. 2019, 10, 1168–1181; (e) Kaur, P.; Hupp, J. T.; Nguyen, S. T. Porous organic polymers in catalysis: opportunities and challenges. ACS Catal. 2011, 1, 819–835; (f) Zhang, Z.; Jia, J.; Zhi, Y.; Ma, S.; Liu, X. Porous organic polymers for light-driven organic transformations. Chem. Soc. Rev. 2022, 51, 2444–2490; (g) Zhou, T.; Huang, X.; Ding, N.; Lin, Z.; Yao, Y.; Guo, J. Porous polyelectrolyte frameworks: synthesis, post-ionization and advanced applications. Chem. Soc. Rev. 2022, 51, 237–267; (h) Zhao, K.; Wang, X.; He, D.; Wang, H.; Qian, B.; Shi, F. Recent development towards alkene hydroformylation catalysts integrating traditional homo-and heterogeneous catalysis. Catal. Sci. Technol. 2022, 12, 4962–4982; (i) Kramer, S.; Bennedsen, N. R.; Kegnæs, S. Porous organic polymers containing active metal centers as catalysts for synthetic organic chemistry. ACS Catal. 2018, 8, 6961–6982; (j) Sun, Q.; Dai, Z.; Meng, X.; Xiao, F. S. Porous polymer catalysts with hierarchical structures. Chem. Soc. Rev. 2015, 44, 6018–6034.
- 15 Iwai, T.; Harada, T.; Hara, K.; Sawamura, M. Threefold cross-linked polystyrene-triphenylphosphane hybrids: mono-P-ligating behavior and catalytic applications for aryl chloride cross-coupling and C(sp3)-H borylation. Angew. Chem. Int. Ed. 2013, 52, 12322–12326.
- 16 Alley, K. A.; Clarkson, A. J.; Uehara, A.; Johnson, J. S. A site-specific synthetic route to substituted inda(box) ligands. Org. Lett. 2023, 25, 9108–9113.
- 17 Matsumoto, H.; Hoshino, Y.; Iwai, T.; Sawamura, M.; Miura, Y. Polystyrene-supported PPh3 in monolithic porous material: Effect of cross-linking degree on coordination mode and catalytic activity in Pd-catalyzed C-C cross-coupling of aryl chlorides. ChemCatChem 2020, 12, 4034–4037.
- 18 Chen, T.; Wang, Z.; Xiao, W.; Yi, C.; Xu, Z. Polystyrene-supported Cu/2,2,6,6-tetramethyl-1-piperidine-N-oxyl catalytic systems constructed by nanoprecipitation and their cooperative catalysis for benzyl alcohol oxidation. ACS Appl. Polym. Mater. 2021, 3, 5171–5179.
- 19 Lu, F. D.; Liu, D.; Zhu, L.; Lu, L. Q.; Yang, Q.; Zhou, Q. Q.; Xiao, W. J. Asymmetric propargylic radical cyanation enabled by dual organophotoredox and copper catalysis. J. Am. Chem. Soc. 2019, 141, 6167–6172.
- 20 Zhang, B.; Li, T. T.; Mao, Z. C.; Jiang, M.; Zhang, Z.; Zhao, K.; Xiao, W. J.; Chen, J. R. Enantioselective cyanofunctionalization of aromatic alkenes via radical anions. J. Am. Chem. Soc. 2024, 146, 1410–1422.
- 21 Xu, H.; Qin, L.; Chen, J.; Wang, Z.; Zhang, W.; Zhang, P.; Tian, W.; Zhang, Y.; Guo, X.; Sun, Z. Toward advanced sodium-ion batteries: A wheel-inspired yolk-shell design for large-volume-change anode materials. J. Mater. Chem. A 2018, 6 , 13153–13163.
- 22(a) Murugesan, K.; Sagadevan, A.; Peng, L.; Savateev, O.; Rueping, M. Recyclable mesoporous graphitic carbon nitride catalysts for the sustainable photoredox catalyzed synthesis of carbonyl compounds. ACS Catal. 2023, 13 , 13414–13422; (b) Gu, H.; Zhang, H.; Wang, X.; Li, Q.; Chang, S.; Huang, Y.; Gao, L.; Cui, Y.; Liu, R.; Dai, W.-L. Robust construction of CdSe nanorods@Ti3C2 MXene nanosheet for superior photocatalytic H2 evolution. Appl. Catal. B-Environ. 2023, 328, 122537.
- 23 Hao, Q.; Li, Z.; Shi, Y.; Li, R.; Li, Y.; Wang, L.; Yuan, H.; Ouyang, S.; Zhang, T. Plasmon-induced radical-radical heterocoupling boosts photodriven oxidative esterification of benzyl alcohol over nitrogen- doped carbon-encapsulated cobalt nanoparticles. Angew. Chem. Int. Ed. 2023, 62, e202312808.
- 24 Li, Y.; Wang, S.; Wang, X. S.; He, Y.; Wang, Q.; Li, Y.; Li, M.; Yang, G.; Yi, J.; Lin, H.; Huang, D.; Li, L.; Chen, H.; Ye, J. Facile top-down strategy for direct metal atomization and coordination achieving a high turnover number in CO2 photoreduction. J. Am. Chem. Soc. 2020, 142, 19259–19267.
- 25 Li, H.; Su, Z.; Hu, S.; Yan, Y. Free-standing and flexible Cu/Cu2O/CuO heterojunction net: a novel material as cost-effective and easily recycled visible-light photocatalyst. Appl. Catal. B-Environ. 2017, 207, 134–142.
- 26
Ma, H.; Zhang, Q. M.; Cheng, G.; Wang, Z.; Zong, Q. S.; Tan, B.; Zhang, C. Heteroatom engineering of hyper-cross-linked polymers for iodine capture. ACS Appl. Polym. Mater. 2020, 3, 209–215.
10.1021/acsapm.0c01047 Google Scholar
- 27 Wan, Y.; Song, F.; Ye, T.; Li, G.; Liu, D.; Lei, Y. Carbonylative Suzuki coupling and alkoxycarbonylation of aryl halides using palladium supported on phosphorus-doped porous organic polymer as an active and robust catalyst. Appl. Organomet. Chem. 2019, 33, e4714.
- 28 Dong, Y.; Chen, Y. Q.; Jv, J. J.; Li, Y.; Li, W. H.; Dong, Y. B. Porous organic polymer with in situ generated palladium nanoparticles as a phase-transfer catalyst for Sonogashira cross-coupling reaction in water. RSC Adv. 2019, 9, 21671–21678.
- 29 Lan, Y.; Yang, C.; Zhang, Y.; An, W.; Xue, H.; Ding, S.; Wang, W. Pyrrolidine-based chiral porous polymers for heterogeneous organocatalysis in water. Polym. Chem. 2019, 10, 3298–3305.
- 30 Li, R. H.; Ding, Z. C.; Li, C. Y.; Chen, J. J.; Zhou, Y. B.; An, X. M.; Zhan, Z. P. Thiophene-alkyne-based CMPs as highly selective regulators for oxidative Heck reaction. Org. Lett. 2017, 19, 4432–4435.




