Photocatalytic [3+2] Cycloaddition of Alkyl/aryl Iodides and Internal Alkynes by Merging Halogen and Hydrogen Atom Transfer
Zhenyu Gu
Key Laboratory of the Ministry of Education for Advanced Catalysis Materials, College of Chemistry and Materials Science, Zhejiang Normal University, 688 Yingbin Road, Jinhua, Zhejiang, 321004 China
Search for more papers by this authorRong Jia
Key Laboratory of the Ministry of Education for Advanced Catalysis Materials, College of Chemistry and Materials Science, Zhejiang Normal University, 688 Yingbin Road, Jinhua, Zhejiang, 321004 China
Search for more papers by this authorTianqing Zeng
Key Laboratory of the Ministry of Education for Advanced Catalysis Materials, College of Chemistry and Materials Science, Zhejiang Normal University, 688 Yingbin Road, Jinhua, Zhejiang, 321004 China
Search for more papers by this authorCorresponding Author
Hanliang Zheng
Key Laboratory of the Ministry of Education for Advanced Catalysis Materials, College of Chemistry and Materials Science, Zhejiang Normal University, 688 Yingbin Road, Jinhua, Zhejiang, 321004 China
E-mail: [email protected], [email protected]Search for more papers by this authorCorresponding Author
Gangguo Zhu
Key Laboratory of the Ministry of Education for Advanced Catalysis Materials, College of Chemistry and Materials Science, Zhejiang Normal University, 688 Yingbin Road, Jinhua, Zhejiang, 321004 China
E-mail: [email protected], [email protected]Search for more papers by this authorZhenyu Gu
Key Laboratory of the Ministry of Education for Advanced Catalysis Materials, College of Chemistry and Materials Science, Zhejiang Normal University, 688 Yingbin Road, Jinhua, Zhejiang, 321004 China
Search for more papers by this authorRong Jia
Key Laboratory of the Ministry of Education for Advanced Catalysis Materials, College of Chemistry and Materials Science, Zhejiang Normal University, 688 Yingbin Road, Jinhua, Zhejiang, 321004 China
Search for more papers by this authorTianqing Zeng
Key Laboratory of the Ministry of Education for Advanced Catalysis Materials, College of Chemistry and Materials Science, Zhejiang Normal University, 688 Yingbin Road, Jinhua, Zhejiang, 321004 China
Search for more papers by this authorCorresponding Author
Hanliang Zheng
Key Laboratory of the Ministry of Education for Advanced Catalysis Materials, College of Chemistry and Materials Science, Zhejiang Normal University, 688 Yingbin Road, Jinhua, Zhejiang, 321004 China
E-mail: [email protected], [email protected]Search for more papers by this authorCorresponding Author
Gangguo Zhu
Key Laboratory of the Ministry of Education for Advanced Catalysis Materials, College of Chemistry and Materials Science, Zhejiang Normal University, 688 Yingbin Road, Jinhua, Zhejiang, 321004 China
E-mail: [email protected], [email protected]Search for more papers by this authorComprehensive Summary
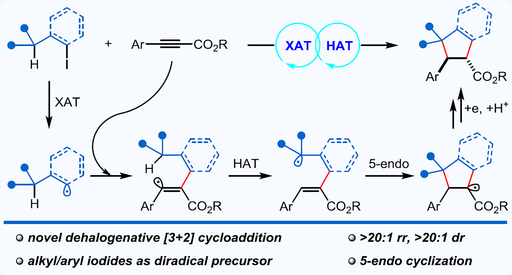
A visible light photocatalytic [3+2] cycloaddition of alkynes with readily accessible organic iodides as the C3 synthon is developed herein. By merging halogen atom transfer (XAT) and hydrogen atom transfer (HAT), alkyl/aryl iodides serve as a formal diradical precursor and add across C-C triple bonds to deliver a number of functionalized cyclopentanes in moderate to high yields with exceptional regio- and diastereoselectivity. A reductive radical-polar crossover mechanism, involving the cascade XAT, radical addition, 1,5-HAT, polar effect-promoted 5-endo annulation, single electron transfer (SET) reduction, and protonation, may account for this unprecedented dehalogenative [3+2] cycloaddition. This work not only expands the repertoire of the traditional RATC methodology, but also provides a robust platform for the expedient assembly of cyclopentanes, a valuable structural motif in the realms of medicinal chemistry and material sciences.
Supporting Information
| Filename | Description |
|---|---|
| cjoc202400366-sup-0001-Supinfo.pdfPDF document, 6.7 MB |
Appendix S1: Supporting Information |
Please note: The publisher is not responsible for the content or functionality of any supporting information supplied by the authors. Any queries (other than missing content) should be directed to the corresponding author for the article.
References
- 1For review, see:(a) Dénès, F.; Beaufils, F.; Renaud, P. Preparation of Five-Membered Rings via the Translocation-Cyclization of Vinyl Radicals. Synlett 2008, 2008, 2389–2399; for selected examples, see: (b) Lamarque, C.; Beaufils, F.; Dénès, F.; Schenk, K.; Renaud, P. Preparation of 5-Membered Rings via Radical Addition-Translocation-Cyclization (RATC) Processes Mediated by Diethyl Thiophosphites. Adv. Synth. Catal. 2011, 353, 1353–1358; (c) Hu, M.; Fan, J.-H.; Liu, Y.; Ouyang, X.-H.; Song, R.-J.; Li, J.-H. Metal-Free Radical [2+2+1] Carbocyclization of Benzene-Linked 1,n-Enynes: Dual C(sp3)-H Functionalization Adjacent to a Heteroatom. Angew. Chem. Int. Ed. 2015, 54, 9577–9580; (d) Qiu, J.-K.; Jiang, B.; Zhu, Y.-L.; Hao, W.-J.; Wang, D.-C.; Sun, J.; Wei, P.; Tu, S.-J.; Li, G. Catalytic Dual 1,1-H-Abstraction/Insertion for Domino Spirocyclizations. J. Am. Chem. Soc. 2015, 137, 8928–8931; (e) Soulard, V.; Dénès, F.; Renaud, P. Effect of Brønsted acids on the thiophenol-mediated radical addition–translocation–cyclization process for the preparation of pyrrolidine derivatives. Free Radical Res. 2016, 50, S2–S5; (f) Gloor, C. S.; Dénès, F.; Renaud, P. Hydrosulfonylation Reaction with Arenesulfonyl Chlorides and Tetrahydrofuran: Conversion of Terminal Alkynes into Cyclopentylmethyl Sulfones. Angew. Chem. Int. Ed. 2017, 56, 13329–13332; (g) Yang, Y.; Daniliuc, C. G.; Studer, A. 1,1,2-Trifunctionalization of Terminal Alkynes by Radical Addition–Translocation–Cyclization–Trapping for the Construction of Highly Substituted Cyclopentanes. Angew. Chem. Int. Ed. 2021, 60, 2145–2148; (h) Le, S.; Bai, Y.; Qiu, J.; Zhang, Z.; Zheng, H.; Zhu, G. Access to Cyclopentenones via Copper- Catalyzed 5-endo Trifluoromethylcarbocyclization of Ynones. Org. Chem. Front. 2022, 9, 4670-4675; (i) Liang, Y.-Q.; Xu, Y.-X.; Cai, Z.-J.; Ji, S.-J. Visible-light photocatalytic radical addition–translocation–cyclization to construct sulfonyl-containing azacycles. Chem. Commun. 2022, 58, 10206–10209; (j) Qiu, J.; Le, S.; Su, J.; Liu, Y.; Zhou, Y.; Zheng, H.; Bai, Y.; Zhu, G. A Diastereoselective Synthesis of Cyclopentanones via Photocatalytic Reductive Alkyltrifluoromethylation of Ynones. Org. Chem. Front. 2022, 9, 5523–5529; (k) Xu, P.; Daniliuc, C. G.; Bergander, K.; Stein, C.; Studer, A. Synthesis of Five-Membered Ring Systems Bearing gem-Difluoroalkenyl and Monofluoroalkenyl Substituents via Radical β-Bromo Fragmentation. ACS Catal. 2022, 12, 11934–11941; (l) Ye, T.; Zhang, F.-L.; Xia, H.-M.; Zhou, X.; Yu, Z.-X.; Wang, Y.-F. Stereoselective hydrogen atom transfer to acyclic radicals: a switch enabling diastereodivergent borylative radical cascades. Nat. Commun. 2022, 13, 426; (m) Zhou, Y.; Qin, Y.; Wang, Q.; Zhang, Z.; Zhu, G. Photocatalytic Sulfonylcarbocyclization of Alkynes Using SEt as a Traceless Directing Group: Access to Cyclopentenes and Indenes. Angew. Chem. Int. Ed. 2022, 61, e202110864; (n) Su, J.; Guo, W.; Liu, Y.; Kong, L.; Zheng, H.; Zhu, G. Cu-catalyzed cascade difluoroalkylation/5-endo cyclization/β-fluorine cleavage of ynones. Chem. Commun. 2023, 59, 1821–1824; (o) Su, J.; Xu, J.; Gu, Z.; Zhou, Y.; Zheng, H.; Zhu, G. Construction of Difluoroalkylated Cyclopentenones through Photocatalytic Difluoroalkylative 5-endo Cyclization Cascades of Ynones. Eur. J. Org. Chem. 2023, 26, e202300432; (p) Wang, C.-L.; Wang, J.; Jin, J.-K.; Li, B.; Phang, Y. L.; Zhang, F.-L.; Ye, T.; Xia, H.-M.; Hui, L.-W.; Su, J.-H.; Fu, Y.; Wang, Y.-F. Boryl radical catalysis enables asymmetric radical cycloisomerization reactions. Science 2023, 382, 1056–1065.
- 2(a) Baldwin, J. E. Rules for Ring Closure. J. Chem. Soc., Chem. Commun. 1976, 734–736; (b) Baldwin, J. E.; Cutting, J.; Dupont, W.; Kruse, L.; Silberman, L.; Thomas, R. C. 5-Endo-Trigonal Reactions: a Disfavoured Ring Closure. J. Chem. Soc., Chem. Commun. 1976, 736–738; (c) Beckwith, A. L. J.; Easton, C. J.; Serelis, A. K. Some guidelines for radical reactions. J. Chem. Soc., Chem. Commun. 1980, 482–483; (d) Chatgilialoglu, C.; Ferreri, C.; Guerra, M.; Timokhin, V.; Froudakis, G.; Gimisis, T. 5-Endo-trig Radical Cyclizations: Disfavored or Favored Processes? J. Am. Chem. Soc. 2002, 124, 10765–10772; (e) Alabugin, I. V.; Timokhin, V. I.; Abrams, J. N.; Manoharan, M.; Abrams, R.; Ghiviriga, I. In Search of Efficient 5-Endo-dig Cyclization of a Carbon- Centered Radical: 40 Years from a Prediction to Another Success for the Baldwin Rules. J. Am. Chem. Soc. 2008, 130, 10984–10995.
- 3(a) Walton, J. C. Non-perfect synchronisation of β-scission with product stabilisation in radical ring-opening reactions. J. Chem. Soc., Perkin Trans. 2 1989, 173–177;
10.1039/P29890000173 Google Scholar(b) Park, S.-U.; Varick, T. R.; Newcomb, M. Acceleration of the 4-exo radical cyclization to a synthetically useful rate. Cyclization of the 2,2-dimethyl-5-cyano-4-pentenyl radical. Tetrahedron Lett. 1990, 31, 2975–2978.
- 4 Cao, Z.; Li, J.; Zhang, G. Photo-induced Copper-Catalyzed Sequential 1,n-HAT Enabling the Formation of Cyclobutanols. Nat. Commun. 2021, 12, 6404.
- 5 Li, J.; Yu, L.; Peng, Y.; Chen, B.; Guo, R.; Ma, X.; Xue, X.-S.; Liu, Y.; Zhang, G. Azetidine Synthesis Enabled by Photo-Induced Copper Catalysis via [3+1] Radical Cascade Cyclization. The Innovation 2022, 3, 100244.
- 6For reviews, see: (a) Ishibashi, H.; Sato, T.; Ikeda, M. 5-Endo-Trig Radical Cyclizations. Synthesis 2002, 695–713; (b) Zheng, H.; Su, J.; Zhou, Y.; Zhu, G. Recent Advances on 5-endo-Trig Radical Cyclization of All-Carbon Systems. Chin. J. Org. Chem. 2022, 42, 4060–4066.
- 7 Le, S.; Li, J.; Feng, J.; Zhang, Z.; Bai, Y.; Yuan, Z.; Zhu, G. [3+2] Cycloaddition of Alkyl Aldehydes and Alkynes Enabled by Photoinduced Hydrogen Atom Transfer. Nat. Commun. 2022, 13, 4734.
- 8 Masuda, Y.; Ikeshita, D.; Higashida, K.; Yoshida, M.; Ishida, N.; Murakami, M.; Sawamura, M. Photocatalytic 1,2-Phosphorus-Migrative [3 + 2] Cycloaddition of Tri(t-butyl)phosphine with Terminal Alkynes. J. Am. Chem. Soc. 2023, 145, 19060–19066.
- 9 Parsaee, F.; Senarathna, M. C.; Kannangara, P. B.; Alexander, S. N.; Arche, P. D. E.; Welin, E. R. Radical philicity and its role in selective organic transformations. Nat. Rev. Chem. 2021, 5, 486–499.
- 10For reviews, see: (a) Juliá, F.; Constantin, T.; Leonori, D. Applications of Halogen-Atom Transfer (XAT) for the Generation of Carbon Radicals in Synthetic Photochemistry and Photocatalysis. Chem. Rev. 2022, 122, 2292–2352; for selected examples, see: (b) Constantin, T.; Juliá, F.; Sheikh, N. S.; Leonori, D. A case of chain propagation: α-aminoalkyl radicals as initiators for aryl radical chemistry. Chem. Sci. 2020, 11, 12822–12828; (c) Constantin, T.; Zanini, M.; Regni, A.; Sheikh, N. S.; Juliá, F.; Leonori, D. Aminoalkyl Radicals as Halogen- Atom Transfer Agents for Activation of Alkyl and Aryl Halides. Science 2020, 1021–1026; (d) Kolahdouzan, K.; Kumar, R.; Gaunt, M. J. Visible-light mediated carbonyl trifluoromethylative amination as a practical method for the synthesis of β-trifluoromethyl tertiary alkylamines. Chem. Sci. 2020, 11, 12089–12094; (e) Zhao, H.; McMillan, A. J.; Constantin, T.; Mykura, R. C.; Juliá, F.; Leonori, D. Merging Halogen-Atom Transfer (XAT) and Cobalt Catalysis to Override E2-Selectivity in the Elimination of Alkyl Halides: A Mild Route toward contra- Thermodynamic Olefins. J. Am. Chem. Soc. 2021, 143, 14806–14813; (f) Zhang, Z.; Górski, B.; Leonori, D. Merging Halogen-Atom Transfer (XAT) and Copper Catalysis for the Modular Suzuki–Miyaura-Type Cross-Coupling of Alkyl Iodides and Organoborons. J. Am. Chem. Soc. 2022, 144, 1986–1992; (g) Caiger, L.; Zhao, H.; Constantin, T.; Douglas, J. J.; Leonori, D. The Merger of Aryl Radical-Mediated Halogen-Atom Transfer (XAT) and Copper Catalysis for the Modular Cross-Coupling-Type Functionalization of Alkyl Iodides. ACS Catal. 2023, 13, 4985–4991; (h) Kostromitin, V. S.; Sorokin, A. O.; Levin, V. V.; Dilman, A. D. Aminals as powerful XAT-reagents: activation of fluorinated alkyl chlorides. Chem. Sci. 2023, 14, 3229–3234; (i) Fan, Q.; Huang, J.; Lin, S.; Chen, Z.-H.; Li, Q.; Yin, B.; Wang, H. Catalyst-Enabled Stereodivergence in Photochemical Atom Transfer Radical Addition (ATRA) of α-Iodoboronic Esters to Alkynes. ACS Catal. 2024, 14, 299–307.
- 11For reviews, see: (a) Stateman, L. M.; Nakafuku, K. M.; Nagib, D. A. Remote C–H Functionalization via Selective Hydrogen Atom Transfer. Synthesis 2018, 50, 1569–1586; (b) Chen, H.; Yu, S. Remote C–C bond formation via visible light photoredox-catalyzed intramolecular hydrogen atom transfer. Org. Biomol. Chem. 2020, 18, 4519–4532; (c) Sarkar, S.; Cheung, K. P. S.; Gevorgyan, V. C–H functionalization reactions enabled by hydrogen atom transfer to carbon-centered radicals. Chem. Sci. 2020, 11, 12974–12993; (d) Guo, W.; Wang, Q.; Zhu, J. Visible Light Photoredox-Catalysed Remote C–H Functionalisation Enabled by 1,5-Hydrogen Atom Transfer (1,5-HAT). Chem. Soc. Rev. 2021, 50, 7359–7377; (e) Yue, B.; Wu, X.; Zhu, C. Recent Advances in Vinyl Radical-Mediated Hydrogen Atom Transfer. Chin. J. Org. Chem. 2022, 42, 458–470; (f) Zhou, Y.; Gu, Z.; Hong, Y.; Chen, H.; Luo, J.; Zheng, H.; Zhu, G. Recent advances in hydrogen atom transfer induced C(sp3)–H functionalizations initiated by radical addition to alkynes. Org. Chem. Front. 2024, 11, 1232–1250.
- 12(a) Pitzer, L.; Schwarz, J. L.; Glorius, F. Reductive radical-polar crossover: traditional electrophiles in modern radical reactions. Chem. Sci. 2019, 10, 8285–8291; (b) Sharma, S.; Singh, J.; Sharma, A. Visible Light Assisted Radical-Polar/Polar-Radical Crossover Reactions in Organic Synthesis. Adv. Synth. Catal. 2021, 363, 3146–3169.




