Divergent Protein Engineering of Transaminase for the Synthesis of Chiral Rivastigmine and Apremilast Precursors†
Langyu Tang
Hubei Engineering Research Center for Biomaterials and Medical Protective Materials, Hubei Key Laboratory of Bioinorganic Chemistry & Materia Medica, School of Chemistry and Chemical Engineering, Huazhong University of Science and Technology, 1037 Luoyu Road, Wuhan, Hubei, 430074 China
Shenzhen Huazhong University of Science and Technology Research Institute, Shenzhen, Guangdong, 518000 China
The authors contribute equally.
Search for more papers by this authorXinjie Yang
Hubei Engineering Research Center for Biomaterials and Medical Protective Materials, Hubei Key Laboratory of Bioinorganic Chemistry & Materia Medica, School of Chemistry and Chemical Engineering, Huazhong University of Science and Technology, 1037 Luoyu Road, Wuhan, Hubei, 430074 China
Longgang Institute of Zhejiang Sci-Tech University, Wenzhou, Zhejiang, 325802 China
The authors contribute equally.
Search for more papers by this authorNingning Sun
Hubei Engineering Research Center for Biomaterials and Medical Protective Materials, Hubei Key Laboratory of Bioinorganic Chemistry & Materia Medica, School of Chemistry and Chemical Engineering, Huazhong University of Science and Technology, 1037 Luoyu Road, Wuhan, Hubei, 430074 China
Search for more papers by this authorGuojiao Wu
Hubei Engineering Research Center for Biomaterials and Medical Protective Materials, Hubei Key Laboratory of Bioinorganic Chemistry & Materia Medica, School of Chemistry and Chemical Engineering, Huazhong University of Science and Technology, 1037 Luoyu Road, Wuhan, Hubei, 430074 China
Search for more papers by this authorYuzhou Wu
Hubei Engineering Research Center for Biomaterials and Medical Protective Materials, Hubei Key Laboratory of Bioinorganic Chemistry & Materia Medica, School of Chemistry and Chemical Engineering, Huazhong University of Science and Technology, 1037 Luoyu Road, Wuhan, Hubei, 430074 China
Search for more papers by this authorCorresponding Author
Fangrui Zhong
Hubei Engineering Research Center for Biomaterials and Medical Protective Materials, Hubei Key Laboratory of Bioinorganic Chemistry & Materia Medica, School of Chemistry and Chemical Engineering, Huazhong University of Science and Technology, 1037 Luoyu Road, Wuhan, Hubei, 430074 China
Shenzhen Huazhong University of Science and Technology Research Institute, Shenzhen, Guangdong, 518000 China
E-mail: [email protected]Search for more papers by this authorLangyu Tang
Hubei Engineering Research Center for Biomaterials and Medical Protective Materials, Hubei Key Laboratory of Bioinorganic Chemistry & Materia Medica, School of Chemistry and Chemical Engineering, Huazhong University of Science and Technology, 1037 Luoyu Road, Wuhan, Hubei, 430074 China
Shenzhen Huazhong University of Science and Technology Research Institute, Shenzhen, Guangdong, 518000 China
The authors contribute equally.
Search for more papers by this authorXinjie Yang
Hubei Engineering Research Center for Biomaterials and Medical Protective Materials, Hubei Key Laboratory of Bioinorganic Chemistry & Materia Medica, School of Chemistry and Chemical Engineering, Huazhong University of Science and Technology, 1037 Luoyu Road, Wuhan, Hubei, 430074 China
Longgang Institute of Zhejiang Sci-Tech University, Wenzhou, Zhejiang, 325802 China
The authors contribute equally.
Search for more papers by this authorNingning Sun
Hubei Engineering Research Center for Biomaterials and Medical Protective Materials, Hubei Key Laboratory of Bioinorganic Chemistry & Materia Medica, School of Chemistry and Chemical Engineering, Huazhong University of Science and Technology, 1037 Luoyu Road, Wuhan, Hubei, 430074 China
Search for more papers by this authorGuojiao Wu
Hubei Engineering Research Center for Biomaterials and Medical Protective Materials, Hubei Key Laboratory of Bioinorganic Chemistry & Materia Medica, School of Chemistry and Chemical Engineering, Huazhong University of Science and Technology, 1037 Luoyu Road, Wuhan, Hubei, 430074 China
Search for more papers by this authorYuzhou Wu
Hubei Engineering Research Center for Biomaterials and Medical Protective Materials, Hubei Key Laboratory of Bioinorganic Chemistry & Materia Medica, School of Chemistry and Chemical Engineering, Huazhong University of Science and Technology, 1037 Luoyu Road, Wuhan, Hubei, 430074 China
Search for more papers by this authorCorresponding Author
Fangrui Zhong
Hubei Engineering Research Center for Biomaterials and Medical Protective Materials, Hubei Key Laboratory of Bioinorganic Chemistry & Materia Medica, School of Chemistry and Chemical Engineering, Huazhong University of Science and Technology, 1037 Luoyu Road, Wuhan, Hubei, 430074 China
Shenzhen Huazhong University of Science and Technology Research Institute, Shenzhen, Guangdong, 518000 China
E-mail: [email protected]Search for more papers by this authorDedicated to the Special Issue of Emerging Investigators in 2024.
Comprehensive Summary
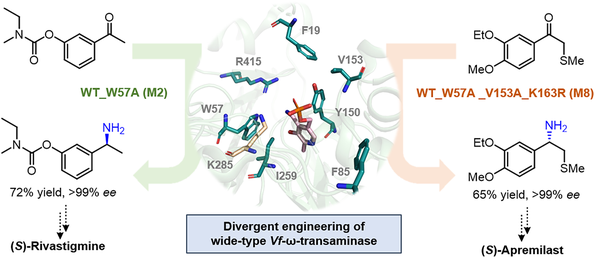
The implementation of divergent protein engineering on the natural transaminase Vf-ω-TA led to the development of two effective mutants (M2 and M8), enabling the enzymatic synthesis of chiral amine precursors of Rivastigmine and Apremilast, respectively. The evolution of the enzymes was guided by crystal structures and a focused mutagenesis strategy, allowing them to effectively address the challenging ketone substrates with significant steric hindrance. Under the optimized reaction parameters, transamination proceeded smoothly in good conversions and with perfect stereochemical control (> 99% ee). These processes utilize inexpensive α-methylbenzylamine as an amine donor and avoid the continuous acetone removal and costly LDH/GDH/NADH systems.
Supporting Information
| Filename | Description |
|---|---|
| cjoc202400351-sup-0001-supinfo.pdfPDF document, 2.3 MB |
Appendix S1: Supporting Information |
Please note: The publisher is not responsible for the content or functionality of any supporting information supplied by the authors. Any queries (other than missing content) should be directed to the corresponding author for the article.
References
- 1(a) Buller, R.; Lutz, S.; Kazlauskas, R. J.; Snajdrova, R.; Moore, J. C.; Bornscheuer, U. T. From nature to industry: Harnessing enzymes for biocatalysis. Science 2023, 382, eadh8615;
(b) O’Connell, A.; Barry, A.; Burke, A. J.; Hutton, A. E.; Bell, E. L.; Green, A. P.; O’Reilly, E. Biocatalysis: landmark discoveries and applications in chemical synthesis. Chem. Soc. Rev. 2024, 53, 2828–2850;
(c) Reetz, M. T.; Qu, G.; Sun, Z. Engineered enzymes for the synthesis of pharmaceuticals and other high-value products. Nat. Synth. 2024, 3, 19–32.
10.1038/s44160-023-00417-0 Google Scholar
- 2For selected reviews, see: (a) Yang, Y.; Arnold, F. H. Navigating the Unnatural Reaction Space: Directed Evolution of Heme Proteins for Selective Carbene and Nitrene Transfer. Acc. Chem. Res. 2021, 54, 1209–1225; (b) Harrison, W.; Huang, X.; Zhao, H. Photobiocatalysis for Abiological Transformations. Acc. Chem. Res. 2022, 55, 1087–1096; (c) Bell, E. L.; Hutton, A. E.; Burke, A. J.; O’Connell, A.; Barry, A.; O’Reilly, E.; Green, A. P. Strategies for designing biocatalysts with new functions. Chem. Soc. Rev. 2024, 53, 2851–2862.
- 3For selected examples from our group and others, see: (a) Huang, X.; Wang, B.; Wang, Y.; Jiang, G.; Feng, J.; Zhao, H. Photoenzymatic enantioselective intermolecular radical hydroalkylation. Nature 2020, 584, 69–74; (b) Fu, Y.; Huang, J.; Wu, Y.; Liu, X.; Zhong, F.; Wang, J. Biocatalytic Cross-Coupling of Aryl Halides with a Genetically Engineered Photosensitizer Artificial Dehalogenase. J. Am. Chem. Soc. 2021, 143, 617–622; (c) Sun, N.; Huang, J.; Qian, J.; Zhou, T.-P.; Guo, J.; Tang, L.; Zhang, W.; Deng, Y.; Zhao, W.; Wu, G.; Liao, R.-Z.; Chen, X.; Zhong, F.; Wu, Y. Enantioselective [2+2]-cycloadditions with triplet photoenzymes. Nature 2022, 611, 715–720; (d) Fu, Y.; Liu, X.; Xia, Y.; Guo, X.; Guo, J.; Zhang, J.; Zhao, W.; Wu, Y.; Wang, J.; Zhong, F. Whole-cell-catalyzed hydrogenation/deuteration of aryl halides with a genetically repurposed photodehalogenase. Chem 2023, 9, 1897–1909; (e) Guo, H.; Sun, N.; Guo, J.; Zhou, T.-P.; Tang, L.; Zhang, W.; Deng, Y.; Liao, R.-Z.; Wu, Y.; Wu, G.; Zhong, F. Catalytic Asymmetric Cyclizative Rearrangement of Anilines and Vicinal Diketones to Access 2,2-Disubstituted Indolin-3-ones, Angew. Chem. Int. Ed. 2023, 62, e202219034; (f) Fu, Y.; Xia, Y.; Xu, Y.; Ye, X.; Xie, S.; Wu, Y.; Zhao, W.; Zhong, F. A Recyclable Supramolecular Photocatalyst for the Chemoselective [2 + 2] Photocycloaddition of Chalcones in Water. ACS Sustainable Chem. Eng. 2023, 11, 17752; (g) Xu, Y.; Chen, H.; Yu, L.; Peng, X.; Zhang, J.; Xing, Z.; Bao, Y.; Liu, A.; Zhao, Y.; Tian, C.; Liang, Y.; Huang, X. A light-driven enzymatic enantioselective radical acylation. Nature 2024, 625, 74–78.
- 4(a) Ghislieri, D.; Turner, N. J. Biocatalytic Approaches to the Synthesis of Enantiomerically Pure Chiral Amines. Top. Catal. 2014, 57, 284–300; (b) Patil, M. D.; Grogan, G.; Bommarius, A.; Yun, H. Oxidoreductase-Catalyzed Synthesis of Chiral Amines. ACS Catal. 2018, 8, 10985–11015.
- 5(a) Nugent, T. C.; El-Shazly, M. Chiral Amine Synthesis–Recent Developments and Trends for Enamide Reduction, Reductive Amination, and Imine Reduction. Adv. Synth. Catal. 2010, 352, 753–819; (b) Trowbridge, A.; Walton, S. M.; Gaunt, M. J. New Strategies for the Transition-Metal Catalyzed Synthesis of Aliphatic Amines. Chem. Rev. 2020, 120, 2613–2692; (c) Yin, Q.; Shi, Y.; Wang, J.; Zhang, X. Direct catalytic asymmetric synthesis of α-chiral primary amines. Chem. Soc. Rev. 2020, 49, 6141–6153.
- 6(a) Guo, F.; Berglund, P. Transaminase biocatalysis: optimization and application. Green Chem. 2017, 19, 333–360; (b) Slabu, I.; Galman, J. L.; Lloyd, R. C.; Turner, N. J. Discovery, Engineering, and Synthetic Application of Transaminase Biocatalysts. ACS Catal. 2017, 7, 8263–8284; (c) Kelly, S. A.; Pohle, S.; Wharry, S.; Mix, S.; Allen, C. C. R.; Moody, T. S.; Gilmore, B. F. Application of ω-Transaminases in the Pharmaceutical Industry. Chem. Rev. 2018, 118, 349–367.
- 7For selected examples, see: (a) Savile, C. K.; Janey, J. M.; Mundorff, E. C.; Moore, J. C.; Tam, S.; Jarvis, W. R.; Colbeck, J. C.; Krebber, A.; Fleitz, F. J.; Brands, J.; Devine, P. N.; Huisman, G. W.; Hughes, G. J. Biocatalytic Asymmetric Synthesis of Chiral Amines from Ketones Applied to Sitagliptin Manufacture. Science 2010, 329, 305–309; (b) Wang, Y.; Feng, J.; Dong, W.; Chen, X.; Yao, P.; Wu, Q.; Zhu, D. Improving Catalytic Activity and Reversing Enantio-Specificity of ω-Transaminase by Semi-Rational Engineering en Route to Chiral Bulky β-Amino Esters. ChemCatChem 2021, 13, 3396–3400; (c) Yang, L.; Zhang, K.; Xu, M.; Xie, Y.; Meng, X.; Wang, H.; Wei, D. Mechanism- Guided Computational Design of ω-Transaminase by Reprograming of High-Energy-Barrier Steps. Angew. Chem. Int. Ed. 2022, 61, e202212555; (d) Ma, Y.; Jiao, X.; Wang, Z.; Mu, H.; Sun, K.; Li, X.; Zhao, T.; Liu, X.; Zhang, N. Engineering a Transaminase for the Efficient Synthesis of a Key Intermediate for Rimegepant. Org. Process Res. Dev. 2022, 26, 1971–1977; (e) Li, C.; Wang, S.; Yang, J.; Yuan, C.; Wang, D.; Shang, D.; O’Brien, E. M. From Chiral Resolution to Diastereoselective Ellman Chemistry to Biocatalysis: Route Evolution for the Efficient Synthesis of the Tetrahydrobenzoazepine Core of BTK Inhibitor BIIB091. Org. Process Res. Dev. 2023, 27, 1463–1473.
- 8(a) Shin, J.-S.; Kim, B.-G. Exploring the Active Site of Amine: Pyruvate Aminotransferase on the Basis of the Substrate Structure-Reactivity Relationship: How the Enzyme Controls Substrate Specificity and Stereoselectivity. J. Org. Chem. 2002, 67, 2848–2853; (b) Cho, B.-K.; Park, H.-Y.; Seo, J.-H.; Kim, J.; Kang, T.-J.; Lee, B.-S.; Kim, B.-G. Redesigning the Substrate Specificity of ω-Aminotransferase for the Kinetic Resolution of Aliphatic Chiral Amines. Biotechnol. Bioeng. 2008, 99, 275–284; (c) Höhne, M.; Schätzle, S.; Jochens, H.; Robins, K.; Bornscheuer, U. T Rational assignment of key motifs for function guides in silico enzyme identification. Nat. Chem. Biol. 2010, 6, 807–813; (d) Fuchs, M.; Farnberger, J.-E.; Kroutil, W. The Industrial Age of Biocatalytic Transamination. Eur. J. Org. Chem. 2015, 6965–6982.
- 9 Spencer, C. M.; Noble, S. Rivastigmine A Review of its Use in Alzheimer's Disease. Drug Aging 1998, 13, 391–411.
- 10 Schett, G.; Sloan, V. S.; Stevens, R. M.; Schafer, P. Apremilast: a novel PDE4 inhibitor in the treatment of autoimmune and inflammatory diseases. Ther. Adv. Musculoskel. Dis. 2010, 2, 271–278.
- 11(a) Boezio, A. A.; Pytkowicz, J.; Côté, A.; Charette, A. B. Asymmetric, Catalytic Synthesis of α-Chiral Amines Using a Novel Bis(phosphine) Monoxide Chiral Ligand. J. Am. Chem. Soc. 2003, 125, 14260–14261; (b) Hu, M.; Zhang, F.-L.; Xie, M.-H. Novel Convenient Synthesis of Rivastigmine. Synth. Commun. 2009, 39, 1527–1533; (c) Mangas-Sánchez, J.; Rodríguez-Mata, M.; Busto, E.; Gotor-Fernández, V.; Gotor, V. Chemoenzymatic Synthesis of Rivastigmine Based on Lipase-Catalyzed Processes. J. Org. Chem. 2009, 74, 5304–5310; (d) Han, K.; Kim, C.; Park, J.; Kim, M.-J. Chemoenzymatic Synthesis of Rivastigmine via Dynamic Kinetic Resolution as a Key Step. J. Org. Chem. 2010, 75, 3105–3108; (e) Rao, R.; Shewalkar, M. P.; Nandipati, R.; Yadav, J. S.; Khagga, M.; Shinde, D. B. General Strategy for Large-Scale Synthesis of (+)Rivastigmine and (+)-NPS R-568. Synth. Commun. 2012, 42, 589–598.
- 12(a) Jana, A.; Zieliński, G. K.; Czarnocka-Śniadała, S.; Grudzień, K.; Podwysocka, D.; Szulc, M.; Kajetanowicz, A.; Grela, K. Synthesis of Substituted β-Functionalised Styrenes by Microwave-Assisted Olefin Cross-Metathesis and Scalable Synthesis of Apremilast. ChemCatChem 2019, 11, 5808–5813; (b) Syu, J.-F.; Gopula, B.; Jian, J.-H.; Li, W.-S.; Kuo, T.-S.; Wu, P.-U.; Henschke, J. P.; Hsieh, M.-C.; Tsai, M.-K.; Wu, H.-L. Asymmetric Synthesis of β-Aryl β-Imido Sulfones Using Rhodium Catalysts with Chiral Diene Ligands: Synthesis of Apremilast. Org. Lett. 2019, 21, 4614–4618; (c) Xiang, C.; Wu, S.; Bornscheuer, U. T. Directed evolution of an amine transaminase for the synthesis of an Apremilast intermediate via kinetic resolution. Bioorg. Med. Chem. 2021, 43, 116271; (d) Heinks, T.; Koopmeiners, S.; Montua, N.; Sewald, N.; Höhne, M.; Bornscheuer, U. T.; von Mollard, G. F. Co-Immobilization of a Multi-Enzyme Cascade: (S)-Selective Amine Transaminases, L-Amino Acid Oxidase and Catalase. ChemBioChem 2023, 24, e202300425.
- 13(a) Fuchs, M.; Koszelewski, D.; Tauber, K.; Kroutila, W.; Faber, K. Chemoenzymatic asymmetric total synthesis of (S)-Rivastigmine using ω-transaminases. Chem. Commun. 2010, 46, 5500–5502; (b) Fuchs, M.; Koszelewski, D.; Tauber, K.; Sattler, J.; Banko, W.; Holzer, A. K.; Pickl, M.; Kroutil, W.; Faber, M. Improved chemoenzymatic asymmetric synthesis of (S)-Rivastigmine. Tetrahedron 2012, 68, 7691–7694.
- 14 Höhne, M.; Bornscheuer, U. T. Biocatalytic Routes to Optically Active Amines. ChemCatChem 2009, 1, 42–51.
- 15(a) Nobili, A.; Steffen-Munsberg, F.; Kohls, H.; Trentin, I.; Schulzke, C.; Hçhne, M.; Bornscheuer, U. T. Engineering the Active Site of the Amine Transaminasefrom Vibrio fluvialisfor the Asymmetric Synthesis of Aryl–Alkyl Amines and Amino Alcohols. ChemCatChem 2015, 7, 757–760; (b) Qu, G.; Li, A.; Acevedo-Rocha, C. G.; Sun, Z.; Reetz, M. T. The crucial role of methodology development in directed evolution of selective enzymes. Angew. Chem. Int. Ed. 2020, 59, 13204−13231; (c) Bao, Y.; Xu, Y.; Huang, X. Focused rational iterative site-specific mutagenesis (FRISM): A powerful method for enzyme engineering. Mol. Catal. 2024, 553, 113755.
- 16(a) Seo, Y.-M.; Mathew, S.; Bea, H.-S.; Khang, Y.-H.; Lee, S.-H.; Kim, B.-G.; Yun, Y. Deracemization of unnatural amino acid: homoalanine using d-amino acid oxidase and ω-transaminase. Org. Biomol. Chem. 2012, 10, 2482–2485; (b) Winter, K. D.; Desmet, T.; Devlamynck, T.; Renterghem, L. V.; Verhaeghe, T.; Pelantová, H.; Křen, V.; Soetaert, W. Biphasic Catalysis with Disaccharide Phosphorylases: Chemoenzymatic Synthesis of α-d-Glucosides Using Sucrose Phosphorylase. Org. Process Res. Dev. 2014, 18, 781–787; (c) Tang, Z.; Li, Q.; Di, J.; Ma, C.; He, Y.-C. An efficient chemoenzymatic cascade strategy for transforming biomass into furfurylamine with lobster shell-based chemocatalyst and mutated ω-transaminase biocatalyst in methyl isobutyl ketone-water. Bioresource Technol. 2023, 369, 128424.
- 17(a) Gao, W.; Lv, H.; Zhang, X. Rh/DuanPhos-Catalyzed Asymmetric Hydrogenation of β-Acetylamino Vinylsulfides: An Approach to Chiral β-Acetylamino Sulfides. Org. Lett. 2017, 19, 2877–2880; (b) Tivadar, T.; Ferene, K.; Janos, H.; Renata, N.; Tihamer, P.; Miklos, T.; Csilla, N. R. Process for the preparation of Apremilast and intermediates by stereoselective reductive amination and enzymic acylation. WO2017059040 A1, 2018.
- 18(a) Vf-ω-TA, PDB: 4E3Q, Midelfort, K. S.; Kumar, R.; Han, S.; Karmilowicz, M. J.; McConnell, K.; Gehlhaar, D. K.; Mistry, A.; Chang, J. S.; Anderson, M.; Villalobos, A.; Minshull, J.; Govindarajan, S.; Wong, J. W. Redesigning and characterizing the substrate specificity and activity of Vibrio fluvialis aminotransferase for the synthesis of imagabalin. Protein Eng. Des. Sel. 2013, 26, 25–33; (b) Cv-ω-TA, PDB: 6S4G, Ruggieri, F.; Campillo-Brocal, J. C.; Chen, S.; Humble, M. S.; Walse, B.; Logan, D. T.; Berglund, P. Insight into the dimer dissociation process of the Chromobacterium violaceum (S)-selective amine transaminase. Sci. Rep. 2019, 9, 16946–16946; (c) Pd-ω-TA, PDB: 4GRX, Rausch, C.; Lerchner, A.; Schiefner, A.; Skerra, A. Crystal structure of the omega-aminotransferase from Paracoccus denitrificans and its phylogenetic relationship with other class III aminotransferases that have biotechnological potential. Proteins 2013, 81, 774–787.




