Vinylogous Fluorine Stabilizing Effect Enables Rational Design of a Novel Donor-Acceptor Cyclopropane and Its Applications in [3+2] Cycloaddition Reaction and Ring-Opening Polymerization†
Yue Li
Hefei National Research Center for Physical Sciences at the Microscale and Department of Polymer Science and Engineering, University of Science and Technology of China, Hefei, Anhui, 230026 China
Search for more papers by this authorCorresponding Author
Dian-Feng Chen
Hefei National Research Center for Physical Sciences at the Microscale and Department of Polymer Science and Engineering, University of Science and Technology of China, Hefei, Anhui, 230026 China
E-mail: [email protected]Search for more papers by this authorYue Li
Hefei National Research Center for Physical Sciences at the Microscale and Department of Polymer Science and Engineering, University of Science and Technology of China, Hefei, Anhui, 230026 China
Search for more papers by this authorCorresponding Author
Dian-Feng Chen
Hefei National Research Center for Physical Sciences at the Microscale and Department of Polymer Science and Engineering, University of Science and Technology of China, Hefei, Anhui, 230026 China
E-mail: [email protected]Search for more papers by this author† Dedicated to the Special Issue of Emerging Investigators in 2024.
Comprehensive Summary
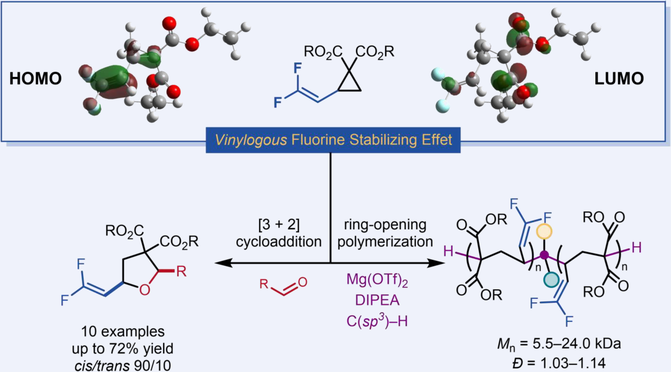
Discovery of unprecedented donor-acceptor patterns can essentially enrich the chemistry of donor-acceptor cyclopropanes. We herein introduce a concept of vinylogous fluorine stabilizing effect, which guides rational design of a novel donor-acceptor cyclopropane employing gem-difluorovinyl group as the electron donor, namely dFVCP. Application of such dFVCPs in a [3+2] cycloaddition with aldehydes and a controlled ring-opening polymerization by a Mg(OTf)2/DIPEA/C(sp3)-H initiator system have been demonstrated, providing direct access to fluorine-containing tetrahydrofurans and all-carbon main-chain polymers.
Supporting Information
| Filename | Description |
|---|---|
| cjoc202400348-sup-0001-supinfo.pdfPDF document, 5.8 MB |
Appendix S1: Supporting Information |
Please note: The publisher is not responsible for the content or functionality of any supporting information supplied by the authors. Any queries (other than missing content) should be directed to the corresponding author for the article.
References
- 1(a) Carson, C. A.; Kerr, M. A. Heterocycles from Cyclopropanes: Applications in Natural Product Synthesis. Chem. Soc. Rev. 2009, 38, 3051–3060; (b) Chen, D. Y.-K.; Pouwer, R. H.; Richard, J.-A. Recent advances in the total synthesis of cyclopropane-containing natural products. Chem. Soc. Rev. 2012, 41, 4631–4642; (c) Talele, T. T. The “Cyclopropyl Fragment” is a Versatile Player that Frequently Appears in Preclinical/Clinical Drug Molecules. J. Med. Chem. 2016, 59, 8712–8756.
- 2 De Meijere, A. Bonding Properties of Cyclopropane and their Chemical Consequences. Angew. Chem. Int. Ed. 1979, 18, 809–826.
- 3For selected examples, see: (a) Stewart, J. M.; Westberg, H. H. Nucleophilic Ring-Opening Additions to 1,1-Disubstituted Cyclopropanes. J. Org. Chem. 1965, 30, 1951–1955; (b) Danishefsky, S.; Dynak, J.; Hatch, E.; Yamamoto, M. Ring Construction through Transpositions of Activated Cyclopropanes. J. Am. Chem. Soc. 1974, 96, 1256–1259; (c) Danshefsky, S. Electrophilic Cyclopropanes in Organic Synthesis. Acc. Chem. Res. 1979, 12, 66–72; (d) Amador, A. G.; Sherbrook, E. M.; Yoon, T. P. Enantioselective Photocatalytic [3+2] Cycloadditions of Aryl Cyclopropyl Ketones. J. Am. Chem. Soc. 2016, 138, 4722–4725.
- 4For selected examples, see: (a) Dunn, J.; Dobbs, A. P. Synthesis and reactions of donor cyclopropanes: efficient routes to cis- and trans- tetrahydrofurans. Tetrahedron 2015, 71, 7386–7414; (b) Woźniak, Ł.; Magagnano, G.; Melchiorre, P. Enantioselective Photochemical Organocascade Catalysis. Angew. Chem. Int. Ed. 2018, 57, 1068–1072; (c) Yang, S.; Wang, L.; Zhang, H.; Liu, C.; Zhang, L.; Wang, X.; Zhang, G.; Li, Y.; Zhang, Q. Copper-Catalyzed Asymmetric Aminocyanation of Arylcyclopropanes for Synthesis of γ-Amino Nitriles. ACS Catal. 2019, 9, 716–721; (d) Wang, M. M.; Waser, J. 1,3-Difunctionalization of Aminocyclopropanes via Dielectrophilic Intermediates. Angew. Chem. Int. Ed. 2019, 58, 13880–13884; (e) Xu, Y.; Gao, H. X.; Pan, C. K.; Shi, Y.; Zhang, C.; Huang, G. P.; Feng, C. Stereoselective Photoredox Catalyzed (3+3) Dipolar Cycloaddition of Nitrone with Aryl Cyclopropane. Angew. Chem. Int. Ed. 2023, 62, e202310671.
- 5 Reissig, H.-U.; Hirsch, E. Donor-Acceptor Substituted Cyclopropanes: Synthesis and Ring Opening to 1,4-Dicarbonyl Compounds. Angew. Chem. Int. Ed. 1980, 19, 813–814.
- 6For excellent reviews, see: (a) Schneider, T. F.; Kaschel, J.; Werz, D. B. A New Golden Age for Donor-Acceptor Cyclopropanes. Angew. Chem. Int. Ed. 2014, 53, 5504–5523; (b) Grover, H. K.; Emmett, M. R.; Kerr, M. A. Carbocycles from Donor-Acceptor Cyclopropanes. Org. Biomol. Chem. 2015, 13, 655–671; (c) Wang, L.; Tang, Y. Asymmetric Ring-opening Reactions of Donor-acceptor Cyclopropanes and Cyclobutanes. Isr. J. Chem. 2016, 56, 463–475; (d) Xia, Y.; Liu, X.; Feng, X. Asymmetric Catalytic Reactions of Donor-Acceptor Cyclopropanes. Angew. Chem. Int. Ed. 2021, 60, 9192–9204; (e) Pirenne, V.; Muriel, B.; Waser, J. Catalytic Enantioselective Ring-Opening Reactions of Cyclopropanes. Chem. Rev. 2021, 121, 227–263.
- 7For selected examples, see: (a) Young, I. S.; Kerr, M. A. A Homo-[3+2]-Dipolar Cycloaddition: The Reaction of Nitrones with Cyclopropanes. Angew. Chem. Int. Ed. 2003, 42, 3023–3026; (b) Pohlhaus, P. D.; Sanders, S. D.; Parsons, A. T.; Li, W.; Johnson, J. S. Scope and Mechanism for Lewis Acid-Catalyzed Cycloadditions of Aldehydes and Donor-Acceptor Cyclopropanes: Evidence for a Stereospecific Intimate Ion Pair Pathway. J. Am. Chem. Soc. 2008, 130, 8642–8650; (c) Kreft, A.; Lücht, A.; Grunenberg, J.; Jones, P. G.; Werz, D. B. Kinetic studies of donor-acceptor cyclopropanes: the influence of structural and electronic properties on the reactivity. Angew. Chem. Int. Ed. 2019, 58, 1955–1959; (d) Garay, G.; Hurtado, J.; Pedrón, M.; García, L.; Reyes, E.; Sánchez-Díez, E.; Tejero, T.; Carrillo, L.; Merino, P.; Vicario, J. L. Organocatalytic Enantioselective Vinylcyclopropane-Cyclopentene (VCP-CP) Rearrangement. Angew. Chem. Int. Ed. 2023, 62, e202302416.
- 8(a) Cho, I.; Ahn, K.-D. Polymerizations of substituted cyclopropanes. II. Anionic polymerization of 1,1-disubstituted 2-vinylcyclopropanes. J. Polym. Sci., Polym. Chem. Ed. 1979, 17, 3183–3191;
(b) Kim, J.-B.; Cho, I. Synthesis and electronic effect of the substituents on anionic ring-opening polymerization of para-substituted phenyl cyclopropanes. Tetrahedron 1997, 53, 15157–15166;
(c) Hayakawa, K.; Matsuoka, S.; Suzuki, M. Ring-opening polymerization of donor–acceptor cyclopropanes catalyzed by Lewis acids. Polym. Chem. 2017, 8, 3841–3847;
(d) Emmerich, A.; Daniliuc, C. G.; Studer, A. Synthesis of Polymers Bearing a Chiral Backbone via Stereospecific Ionic Ring-Opening Polymerization of Chiral Donor-Acceptor Cyclopropanes. Macromol. Rapid Commun. 2021, 42, 2100030;
(e) Li, Y.-Y.; Xie, T.; Zhu, L.; Zheng, S.-Q.; Chen, D.-F.; Gong, L.-Z. Organo–metal cooperative catalysis for C(sp3)–H alkylation polymerization. Nat. Synth. 2023, 2, 1232–1242.
10.1038/s44160-023-00389-1 Google Scholar
- 9For a review, see: (a) Studer, A.; Curran, D. P. Catalysis of radical reactions: a radical chemistry perspective. Angew. Chem. Int. Ed. 2016, 55, 58–102. For selected examples, see: (b) Zhang, H.; Curran, D. P. A Short Total Synthesis of (±)-Epimeloscine and (±)-Meloscine Enabled by a Cascade Radical Annulation of a Divinylcyclopropane. J. Am. Chem. Soc. 2011, 133, 10376–10378; (c) Hashimoto, T.; Kawamata, Y.; Maruoka, K. An Organic Thiyl Radical Catalyst for Enantioselective Cyclization. Nat. Chem. 2014, 6, 702–705; (d) Chen, D.-F.; Boyle, B. M.; McCarthy, B. G.; Lim, C.-H.; Miyake, G. M. Controlling Polymer Composition in Organocatalyzed Photoredox Radical Ring-Opening Polymerization of Vinylcyclopropanes. J. Am. Chem. Soc. 2019, 141, 13268−13277; (e) Chen, D.-F.; Chrisman, C. H.; Miyake, G. M. Bromine Radical Catalysis by Energy Transfer Photosensitization. ACS Catal. 2020, 10, 2609–2614; (f) Zhao, Q.-Q.; Zhou, X.-S.; Xu, S.-H.; Wu, Y.-L.; Xiao, W.-J.; Chen, J.-R. Visible-Light-Driven Nitrogen Radical- Catalyzed [3 + 2] Cyclization of Vinylcyclopropanes and N-Tosyl Vinylaziridines with Alkenes. Org. Lett. 2020, 22, 2470–2475; (g) Archer, G.; Cavalère, P.; Médebielle, M.; Merad, J. Angew. Chem. Int. Ed. 2022, 61, e202205596; (h) Wu, B.; Ding, Q.-J.; Wang, Z.-L.; Zhu, R. Alkyne Polymers from Stable Butatriene Homologues: Controlled Radical Polymerization of Vinylidenecyclopropanes. J. Am. Chem. Soc. 2023, 145, 2045−2051.
- 10(a) Wenkert, E.; Alonso, M. E.; Buckwalter, B. L.; Chou, J. K. A Method of Synthesis of β-Methylfurans and α-Methylene and β-Methylene γ-Lactones. Two Menthofuran Syntheses. J. Am. Chem. Soc. 1977, 99, 4778–4782; (b) Reissig, H. U.; Hirsch, E. Donor-Acceptor Substituted Cyclopropanes: Synthesis and Ring Opening to 1,4-Dicarbonyl Compounds. Angew. Chem. Int. Ed. 1980, 19, 813–814; (c) Horiguchi, Y.; Suehiro, A.; Kuwajima, I. Diastereoselective [3+2] Cycloaddition of Methyl 2-Phenylthiocyclopropyl Ketone with Enol Silyl Ethers: Synthesis of Functionalized Cyclopentanes. Tetrahedron Lett. 1993, 34, 6077–6080.
- 11(a) Racine, S.; de Nanteuil, F.; Serrano, E.; Waser, J. Synthesis of (Carbo)nucleoside Analogues by [3+2] Annulation of Aminocyclopropanes. Angew. Chem. Int. Ed. 2014, 53, 8484–8487; (b) de Nanteuil, F.; Serrano, E.; Perrotta, D.; Waser, J. Dynamic Kinetic Asymmetric [3+2] Annulation Reactions of Aminocyclopropanes. J. Am. Chem. Soc. 2014, 136, 6239–6242; (c) De Nanteuil, F.; De Simone, F.; Frei, R.; Benfatti, F.; Serrano, E.; Waser, J. Cyclization and annulation reactions of nitrogen-substituted cyclopropanes and cyclobutanes. Chem. Commun. 2014, 50, 10912–10928; (d) Suleymanov, A. A.; Le Du, E.; Dong, Z.; Muriel, B.; Scopelliti, R.; Fadaei-Tirani, F.; Waser, J.; Severin, K. Triazene-Activated Donor-Acceptor Cyclopropanes: Ring- Opening and (3+2) Annulation Reactions. Org. Lett. 2020, 22, 4517–4522.
- 12(a) Yadav, V. K.; Balamurugan, R. Silicon-assisted ring opening of donor-acceptor substituted cyclopropanes. An expedient entry to substituted dihydrofurans. Org. Lett. 2001, 3, 2717–2719; (b) Agrawal, D.; Yadav, V. K. Silylmethyl-substituted cyclopropyl and other strained ring systems: cycloaddition with dipolarophiles. Chem. Commun. 2008, 48, 6471–6488.
- 13 O’Hagan, D. Understanding organofluorine chemistry. An introduction to the C–F bond. Chem. Soc. Rev. 2008, 37, 308–319.
- 14 Ni, C.; Hu, J. The unique fluorine effects in organic reactions: recent facts and insights into fluoroalkylations. Chem. Soc. Rev. 2016, 45, 5441–5454.
- 15 Blint, R. J.; McMahon, T. B.; Beauchamp, J. L. Gas-phase Ion Chemistry of Fluoromethanes by Ion Cyclotron Resonance Spectroscopy. New Techniques for the Determination of Carbonium Ion Stabilities. J. Am. Chem. Soc. 1974, 96, 1269–1278.
- 16 Liu, H.; Tian, L.; Wang, H.; Li, Z.-Q.; Zhang, C.; Xue, F.; Feng, C. A Novel Type of Donor–Acceptor Cyclopropane with Fluorine as the Donor: (3+2)-Cycloadditions with Carbonyls. Chem. Sci. 2022, 13, 2686–2691.
- 17For reviews, see: (a) Vitale, A.; Bongiovanni, R.; Ameduri, B. Fluorinated Oligomers and Polymers in Photopolymerization. Chem. Rev. 2015, 115, 8835–8866; (b) Ameduri, B. Fluoropolymers: The Right Material for the Right Applications. Chem. Eur. J. 2018, 24, 18830–18841. For selected examples, see: (c) Gong, H.; Zhao, Y.; Shen, X.; Lin, J.; Chen, M. Organo-catalyzed Photo-Controlled Radical Polymerization of Semi-Fluori-nated (Meth)acrylates Driven by Visible Light. Angew. Chem. Int. Ed. 2018, 57, 333−337; (d) Gong, H.; Gu, Y.; Zhao, Y.; Quan, Q.; Han, S.; Chen, M. Precise Synthesis of Ultra-High-Molecular-Weight Fluoropolymers Enabled by Chain-Transfer-Agent Differentiation under Visible-Light Irradiation. Angew. Chem. Int. Ed. 2020, 59, 919−927; (e) Jiang, K.; Han, S.; Ma, M.; Zhang, L.; Zhao, Y.; Chen, M. Photoorganocatalyzed Reversible-Deactivation Alternating Copolymerization of Chlorotrifluoroethylene and Vinyl Ethers under Ambient Conditions: Facile Access to Main-Chain Fluorinated Copolymers. J. Am. Chem. Soc. 2020, 142, 7108−7115.
- 18(a) Pohlhaus, P. D.; Johnson, J. S. Highly Diastereoselective Synthesis of Tetrahydrofurans via Lewis Acid-Catalyzed Cyclopropane/Aldehyde Cycloadditions. J. Org. Chem. 2005, 70, 1057–1059; (b) Pohlhaus, P. D.; Johnson, J. S. Enantiospecific Sn(II)- and Sn(IV)-Catalyzed Cycloadditions of Aldehydes and Donor-Acceptor Cyclopropanes. J. Am. Chem. Soc. 2005, 127, 16014–16015.




