Chemoenzymatic Synthesis of N,N,N-Trimethyl-D-Glucosamine Chitotriomycin and Its Analogues
Jianghua Li
Key Laboratory of Marine Drugs of Ministry of Education, Shandong Key Laboratory of Glycoscience and Glycotechnology, School of Medicine and Pharmacy, Ocean University of China, Qingdao, Shandong, 266003 China
Search for more papers by this authorYan Zhang
Key Laboratory of Marine Drugs of Ministry of Education, Shandong Key Laboratory of Glycoscience and Glycotechnology, School of Medicine and Pharmacy, Ocean University of China, Qingdao, Shandong, 266003 China
Search for more papers by this authorCorresponding Author
Zhifei Hu
Key Laboratory of Marine Drugs of Ministry of Education, Shandong Key Laboratory of Glycoscience and Glycotechnology, School of Medicine and Pharmacy, Ocean University of China, Qingdao, Shandong, 266003 China
Laboratory for Marine Drugs and Bioproducts, Laoshan Laboratory, Qingdao, Shandong, 266237 China
E-mail: [email protected]; [email protected]Search for more papers by this authorJinfeng Ye
Key Laboratory of Marine Drugs of Ministry of Education, Shandong Key Laboratory of Glycoscience and Glycotechnology, School of Medicine and Pharmacy, Ocean University of China, Qingdao, Shandong, 266003 China
Search for more papers by this authorCorresponding Author
Hongzhi Cao
Key Laboratory of Marine Drugs of Ministry of Education, Shandong Key Laboratory of Glycoscience and Glycotechnology, School of Medicine and Pharmacy, Ocean University of China, Qingdao, Shandong, 266003 China
Laboratory for Marine Drugs and Bioproducts, Laoshan Laboratory, Qingdao, Shandong, 266237 China
E-mail: [email protected]; [email protected]Search for more papers by this authorJianghua Li
Key Laboratory of Marine Drugs of Ministry of Education, Shandong Key Laboratory of Glycoscience and Glycotechnology, School of Medicine and Pharmacy, Ocean University of China, Qingdao, Shandong, 266003 China
Search for more papers by this authorYan Zhang
Key Laboratory of Marine Drugs of Ministry of Education, Shandong Key Laboratory of Glycoscience and Glycotechnology, School of Medicine and Pharmacy, Ocean University of China, Qingdao, Shandong, 266003 China
Search for more papers by this authorCorresponding Author
Zhifei Hu
Key Laboratory of Marine Drugs of Ministry of Education, Shandong Key Laboratory of Glycoscience and Glycotechnology, School of Medicine and Pharmacy, Ocean University of China, Qingdao, Shandong, 266003 China
Laboratory for Marine Drugs and Bioproducts, Laoshan Laboratory, Qingdao, Shandong, 266237 China
E-mail: [email protected]; [email protected]Search for more papers by this authorJinfeng Ye
Key Laboratory of Marine Drugs of Ministry of Education, Shandong Key Laboratory of Glycoscience and Glycotechnology, School of Medicine and Pharmacy, Ocean University of China, Qingdao, Shandong, 266003 China
Search for more papers by this authorCorresponding Author
Hongzhi Cao
Key Laboratory of Marine Drugs of Ministry of Education, Shandong Key Laboratory of Glycoscience and Glycotechnology, School of Medicine and Pharmacy, Ocean University of China, Qingdao, Shandong, 266003 China
Laboratory for Marine Drugs and Bioproducts, Laoshan Laboratory, Qingdao, Shandong, 266237 China
E-mail: [email protected]; [email protected]Search for more papers by this authorComprehensive Summary
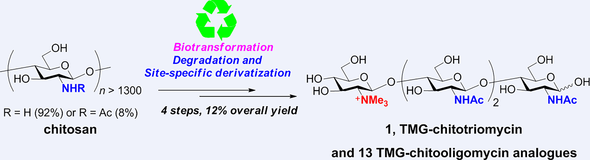
N,N,N-Trimethyl-D-glucosamine (TMG)-chitotriomycin, a naturally occurring chitin related oligosaccharide, is a specific β-N-acetylhexosaminidases (HexNAcases) inhibitor for insects and fungi. Although TMG-chitoriomycin holds great promise as a novel class insecticide and fungicide, the limited accessibility of TMG-chitotriomycin prevents its further biological evaluation. We report herein a simple and eco-friendly chemoenzymatic approach for the efficient synthesis of TMG-chitotriomycin and its analogues. In this strategy, the readily available chitosan was enzymatically hydrolyzed and chemically N-acetylated to afford the chitooligosaccharides ranging from disaccharide to hexasaccharide. These chitooligosaccharides were selectively deacetylated by two different chitin deacetylases followed by chemical N-trimethylation to obtain the desired TMG-chitotriomycin and a total of 13 TMG-chitotriomycin analogues in the longest linear sequence of 4 steps in over 12% total yields.
Supporting Information
| Filename | Description |
|---|---|
| cjoc202400345-sup-0001-supinfo.pdfPDF document, 14.3 MB |
Appendix S1: Supporting Information |
Please note: The publisher is not responsible for the content or functionality of any supporting information supplied by the authors. Any queries (other than missing content) should be directed to the corresponding author for the article.
References
- 1 Usuki, H.; Nitoda, T.; Ichikawa, M.; Yamaji, N.; Iwashita, T.; Komura, H.; Kanzaki, H. TMG-chitotriomycin, an enzyme inhibitor specific for insect and fungal bate-N-acetylglucosaminidases, produced by actinomycete Streptomyces anulatus NBRC 13369. J. Am. Chem. Soc. 2008, 130, 4146–4152.
- 2 Yang, Y.; Li, Y.; Yu, B. Total synthesis and structural revision of TMG- chitotriomycin, a specific inhibitor of insect and fungal bate-N-acetylglucosaminidases. J. Am. Chem. Soc. 2009, 131, 12076–12077.
- 3 Yang, Y.; Liu, T.; Yang, Y.; Wu, Q.; Yang, Q.; Yu, B. Synthesis, evaluation, and mechanism of N,N,N-trimethyld-glucosamine-(1→4)-chitooligosaccharides as selective inhibitors of glycosyl hydrolase family 20 bate-N-acetyl-dhexosaminidases. ChemBioChem 2011, 12, 457–467.
- 4 Shiota, H.; Kanzaki, H.; Hatanaka, T.; Nitoda, T. TMG-chitotriomycin as a probe for the prediction of substrate specificity of bate-N-acetylhexosaminidases. Carbohydr. Res. 2013, 375, 29–34.
- 5 Liu, T.; Zhang, H.; Liu, F.; Wu, Q.; Shen, X.; Yang, Q. Structural determinants of an insect bate-N-acetyl-D-hexosaminidase specialized as a chitinolytic Enzyme. J. Biol. Chem. 2011, 286, 4049–4058.
- 6 Usuki, H.; Yamamoto, Y.; Kumagai, Y.; Nitoda, T.; Kanzaki, H.; Hatanaka, T. MS/MS fragmentation-guided search of TMG-chitooligomycins and their structure–activity relationship in specific bate-N- acetyl-glucosaminidase inhibition. Org. Biomol. Chem. 2011, 9, 2943–2951.
- 7 Yang, Y.; Yu, B. Recent advances in the synthesis of chitooligosaccharides and congeners. Tetrahedron 2014, 70, 1023–1046.
- 8 Isoda, Y.; Sasaki, N.; Kitamura, K.; Takahashi, S.; Manmode, S.; Takeda-Okuda, N.; Tamura, J.; Nokami, T.; Itoh, T. Total synthesis of TMG-chitotriomycin based on an automated electrochemical assembly of a disaccharide building block. Beilstein J. Org. Chem. 2017, 13, 919–924.
- 9 Nokami, T.; Isoda, Y.; Sasaki, N.; Takaiso, A.; Hayase, S.; Itoh, T.; Hayashi, R.; Shimizu, A.; Yoshida, J. Automated electrochemical assembly of the protected potential TMG-chitotriomycin precursor based on rational optimization of the carbohydrate building block. Org. Lett. 2015, 17, 1525–1528.
- 10 He, H.; Xu, L.; Sun, R.; Zhang, Y.; Huang, Y.; Chen, Z.; Li, P.; Yang, R.; Xiao, G. An orthogonal and reactivity-based one-pot glycosylation strategy for both glycan and nucleoside synthesis: access to TMG- chitotriomycin, lipochitooligosaccharides and capuramycin. Chem. Sci. 2021, 12, 5143–5151.
- 11 Zhang, Y.; Xiao, G. Chemical synthesis of TMG-chitotriomycin. J. Carbohydr. Chem. 2021, 40, 327–338.
- 12 Ding, H.; Lyu, J.; Zhang, X.-L.; Xiao, X.; Liu, X.-W. Efficient and versatile formation of glycosidic bonds via catalytic strain-release glycosylation with glycosyl ortho−2,2-dimethoxycarbonylcyclopropylbenzoate donors. Nat. Commun. 2023, 14, 4010–4011.
- 13 Gong, C.; Ju, Z.; Sheng, K.; Zhang, X. Efficient conversion of chitin into 5-hydroxymethylfurfural via a simple formylation step under mild conditions. Green Chem. 2023, 25, 4781–4792.
- 14 Yan, N.; Chen, X. Don't waste seafood waste. Nature 2015, 524, 155–157.
- 15 Aam, B. B.; Heggset, E. B.; Norberg, A. L.; Sørlie, M.; Vårum, K. M.; Eijsink, V. G. H. Production of chitooligosaccharides and their potential applications in medicine. Mar. Drugs 2010, 8, 1482–1517.
- 16 Zhang, J.; Cao, H.; Li, S.; Zhao, Y.; Wang, W.; Xu, Q.; Du, Y.; Yin, H. Characterization of a new family 75 chitosanase from Aspergillus sp. W-2. Int. J. Biol. Macromol. 2015, 81, 362–369.
- 17 Despras, G.; Alix, A.; Urban, D.; Vauzeilles, B.; Beau, J.-M. From chitin to bioactive chitooligosaccharides and conjugates: access to lipochitooligosaccharides and the TMG-chitotriomycin. Angew. Chem. Int. Ed. 2014, 53, 11912–11916.
- 18 Cottaz, S.; Samain, E. Genetic engineering of Escherichia coli for the production of NI,NII-diacetylchitobiose (chitinbiose) and its utilization as a primer for the synthesis of complex carbohydrates. Metab. Eng. 2005, 7, 311–317.
- 19 Samain, E.; Chazalet, V.; Geremia, R. A. Production of O-acetylated and sulfated chitooligosaccharides by recombinant Escherichia coli strains harboring different combinations of nod genes. J. Biotechnol. 1999, 72, 33–47.
- 20 Samain, E.; Drouillard, S.; Heyraud, A.; Driguez, H.; Geremia, R. A. Gram-scale synthesis of recombinant chitooligosaccharides in Escherichia coli. Carbohydr. Res. 1997, 302, 35–42.
- 21 Halila, S.; Samain, E.; Vorgias, C. E.; Armand, S. A straightforward access to TMG-chitooligomycins and their evaluation as bate-N-acetylhexosaminidase inhibitors. Carbohydr. Res. 2013, 368, 52–56.
- 22 Yan, J.; Li, J.; Hu, Z.; Ma, X.; Li, Y.; Hu, X.; Ye, J.; Wang, W.; Wan, R.; Cao, H. Enzymatic synthesis of sialyl lactosamine grafted chitooligosaccharides. Chin. J. Chem. 2023, 41, 1299–1304.
- 23 Li, X.; Wang, L.-X.; Wang, X.; Roseman, S. The chitin catabolic cascade in the marine bacterium Vibrio cholerae: Characterization of a unique chitin oligosaccharide deacetylase. Glycobiology 2007, 17, 1377–1387.
- 24 Hirano, T.; Sugiyama, K.; Sakaki, Y.; Hakamata, W.; Park, S.-Y.; Nishio, T. Structure-based analysis of domain function of chitin oligosaccharide deacetylase from Vibrio parahaemolyticus. FEBS Lett. 2014, 589, 145–151.
- 25 John, M.; Röhrig, H.; Schmidt, J.; Wieneke, U.; Schell, J. Rhizobium NodB protein involved in nodulation signal synthesis is a chitooligosaccharide deacetylase. Biochemistry 1993, 90, 625–629.
- 26 Chambon, R.; Pradeau, S.; Fort, S.; Cottaz, S.; Armand, S. High yield production of Rhizobium NodB chitin deacetylase and its use for in vitro synthesis of lipo-chitinoligosaccharide precursors. Carbohydr. Res. 2017, 442, 25–30.




