On-surface Synthesis of Multiple Non-benzenoid Carbohelicenes Fused with Fluorene Unit(s)
Wei Xiong
Faculty of Materials Science and Engineering, Kunming University of Science and Technology, Kunming, Yunnan, 650093 China
These authors contribute equally to this work.
Search for more papers by this authorXi Geng
Faculty of Materials Science and Engineering, Kunming University of Science and Technology, Kunming, Yunnan, 650093 China
These authors contribute equally to this work.
Search for more papers by this authorCorresponding Author
Jianchen Lu
Faculty of Materials Science and Engineering, Kunming University of Science and Technology, Kunming, Yunnan, 650093 China
These authors contribute equally to this work.
E-mail: [email protected]; [email protected]; [email protected]Search for more papers by this authorGefei Niu
Faculty of Materials Science and Engineering, Kunming University of Science and Technology, Kunming, Yunnan, 650093 China
Search for more papers by this authorBoyu Fu
Faculty of Materials Science and Engineering, Kunming University of Science and Technology, Kunming, Yunnan, 650093 China
Search for more papers by this authorYi Zhang
Faculty of Materials Science and Engineering, Kunming University of Science and Technology, Kunming, Yunnan, 650093 China
Search for more papers by this authorShicheng Li
Faculty of Materials Science and Engineering, Kunming University of Science and Technology, Kunming, Yunnan, 650093 China
Search for more papers by this authorYuhang Yang
Faculty of Materials Science and Engineering, Kunming University of Science and Technology, Kunming, Yunnan, 650093 China
Search for more papers by this authorNianqiang Li
Faculty of Materials Science and Engineering, Kunming University of Science and Technology, Kunming, Yunnan, 650093 China
Search for more papers by this authorCorresponding Author
Lei Gao
Faculty of Science, Kunming University of Science and Technology, Kunming, Yunnan, 650093 China
E-mail: [email protected]; [email protected]; [email protected]Search for more papers by this authorCorresponding Author
Jinming Cai
Faculty of Materials Science and Engineering, Kunming University of Science and Technology, Kunming, Yunnan, 650093 China
Southwest United Graduate School, Kunming, Yunnan, 650093 China
E-mail: [email protected]; [email protected]; [email protected]Search for more papers by this authorWei Xiong
Faculty of Materials Science and Engineering, Kunming University of Science and Technology, Kunming, Yunnan, 650093 China
These authors contribute equally to this work.
Search for more papers by this authorXi Geng
Faculty of Materials Science and Engineering, Kunming University of Science and Technology, Kunming, Yunnan, 650093 China
These authors contribute equally to this work.
Search for more papers by this authorCorresponding Author
Jianchen Lu
Faculty of Materials Science and Engineering, Kunming University of Science and Technology, Kunming, Yunnan, 650093 China
These authors contribute equally to this work.
E-mail: [email protected]; [email protected]; [email protected]Search for more papers by this authorGefei Niu
Faculty of Materials Science and Engineering, Kunming University of Science and Technology, Kunming, Yunnan, 650093 China
Search for more papers by this authorBoyu Fu
Faculty of Materials Science and Engineering, Kunming University of Science and Technology, Kunming, Yunnan, 650093 China
Search for more papers by this authorYi Zhang
Faculty of Materials Science and Engineering, Kunming University of Science and Technology, Kunming, Yunnan, 650093 China
Search for more papers by this authorShicheng Li
Faculty of Materials Science and Engineering, Kunming University of Science and Technology, Kunming, Yunnan, 650093 China
Search for more papers by this authorYuhang Yang
Faculty of Materials Science and Engineering, Kunming University of Science and Technology, Kunming, Yunnan, 650093 China
Search for more papers by this authorNianqiang Li
Faculty of Materials Science and Engineering, Kunming University of Science and Technology, Kunming, Yunnan, 650093 China
Search for more papers by this authorCorresponding Author
Lei Gao
Faculty of Science, Kunming University of Science and Technology, Kunming, Yunnan, 650093 China
E-mail: [email protected]; [email protected]; [email protected]Search for more papers by this authorCorresponding Author
Jinming Cai
Faculty of Materials Science and Engineering, Kunming University of Science and Technology, Kunming, Yunnan, 650093 China
Southwest United Graduate School, Kunming, Yunnan, 650093 China
E-mail: [email protected]; [email protected]; [email protected]Search for more papers by this authorComprehensive Summary
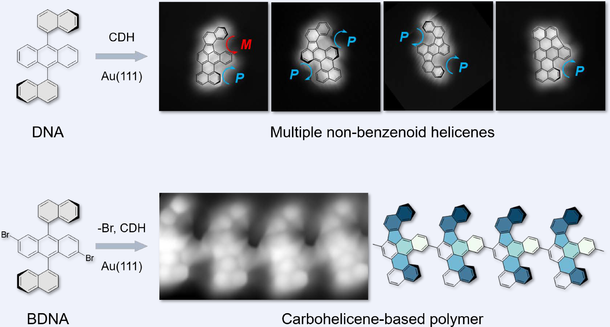
Carbohelicenes have garnered considerable attention for their inherent chirality and structural flexibility. Increasing multi-helicity and incorporating non-six-membered rings to substitute benzenoid rings within helicenes are effective strategies for introducing unique photoelectric properties. Despite the disclosure of numerous helicenes, the inaccessible precursors and the lack of synthetic routes pose a challenge in achieving desired helicene structures fused with non-benzenoid rings. Herein, we report the synthesis of multiple non-benzenoid carbohelicenes fused with fluorene unit(s) through intramolecular cyclodehydrogenation of 9,10-di(naphthalen-1- yl)anthracene on Au(111) surface. Two potential cyclodehydrogenation manners between naphthyl and anthracene lead to the formation of fluorene-fused [5]helicene and [4]helicene moiety. Consequently, a total of four stable products were observed. The atomic topographies of products are characterized by bond-resolving scanning tunneling microscopy. The chiral helicity of targeted products can be switched by tip manipulation. Density-functional-theory calculations unveils the reaction pathway of four products. The comparative analysis of their respective energy barriers exhibits a correlation with the experimentally determined yields. Furthermore, we synthesize the polymer chains incorporating non-benzenoid carbohelicenes via the Ullmann reaction of 2,6-dibromo-9,10-di(1-naphthyl)anthracene precursors. Our work proposes a synthetic methodology for several novel helicene-like structures fused with fluorene units and the polymer bearing helicene subunits, thus highlighting the immense potential of these compounds in the application fields of luminescent electronic devices.
Supporting Information
| Filename | Description |
|---|---|
| cjoc202400341-sup-0001-supinfo.pdfPDF document, 1.8 MB |
Appendix S1: Supporting Information |
Please note: The publisher is not responsible for the content or functionality of any supporting information supplied by the authors. Any queries (other than missing content) should be directed to the corresponding author for the article.
References
- 1 Gingras, M.; Félix, G.; Peresutti, R. One hundred years of helicene chemistry. Part 2: stereoselective syntheses and chiral separations of carbohelicenes. Chem. Soc. Rev. 2013, 42, 1007–1050.
- 2 Biet, T.; Martin, K.; Hankache, J.; Hellou, N.; Hauser, A.; Bürgi, T.; Vanthuyne, N.; Aharon, T.; Caricato, M.; Crassous, J.; Avarvari, N. Triggering Emission with the Helical Turn in Thiadiazole-Helicenes. Chem. Eur. J. 2017, 23, 437–446.
- 3 Brand, M.; Norman, P. Nontrivial spectral band progressions in electronic circular dichroism spectra of carbohelicenes revealed by linear response calculations. Phys. Chem. Chem. Phys. 2022, 24, 19321–19332.
- 4 Hong, J.; Xiao, X.; Liu, H.; Dmitrieva, E.; Popov, A. A.; Yu, Z.; Li, M.-D.; Ohto, T.; Liu, J.; Narita, A.; Liu, P.; Tada, H.; Cao, X.-Y.; Wang, X.-Y.; Zou, Y.; Müllen, K.; Hu, Y. Controlling the Emissive, Chiroptical, and Electrochemical Properties of Double [7] Helicenes through Embedded Aromatic Rings. Chem. Eur. J. 2022, 28, e202202243.
- 5 Hu, Y.; Wang, X.-Y.; Peng, P.-X.; Wang, X.-C.; Cao, X.-Y.; Feng, X.; Müllen, K.; Narita, A. Benzo-Fused Double [7]Carbohelicene: Synthesis, Structures, and Physicochemical Properties. Angew. Chem. Int. Ed. 2017, 56, 3374–3378.
- 6 Shiotari, A.; Tanaka, K.; Nakae, T.; Mori, S.; Okujima, T.; Uno, H.; Sakaguchi, H.; Sugimoto, Y. Chiral Discrimination and Manipulation of Individual Heptahelicene Molecules on Cu(001) by Noncontact Atomic Force Microscopy. J. Phys. Chem. C 2018, 122, 4997–5003.
- 7 Stetsovych, O.; Mutombo, P.; Švec, M.; Šámal, M.; Nejedlý, J.; Císařová, I.; Vázquez, H.; Moro-Lagares, M.; Berger, J.; Vacek, J.; Stará, I. G.; Starý, I.; Jelínek, P. Large Converse Piezoelectric Effect Measured on a Single Molecule on a Metallic Surface. J. Am. Chem. Soc. 2018, 140, 940–946.
- 8 Li, C.; Yang, Y.; Miao, Q. Recent Progress in Chemistry of Multiple Helicenes. Chem. Asian J. 2018, 13, 884–894.
- 9 Zhang, L.; Song, I.; Ahn, J.; Han, M.; Linares, M.; Surin, M.; Zhang, H.-J.; Oh, J. H.; Lin, J. π-Extended perylene diimide double-heterohelicenes as ambipolar organic semiconductors for broadband circularly polarized light detection. Nat. Commun. 2021, 12, 142.
- 10 Márquez, I. R.; Fuentes, N.; Cruz, C. M.; Puente-Muñoz, V.; Sotorrios, L.; Marcos, M. L.; Choquesillo-Lazarte, D.; Biel, B.; Crovetto, L.; Gómez-Bengoa, E.; González, M. T.; Martin, R.; Cuerva, J. M.; Campaña, A. G. Versatile synthesis and enlargement of functionalized distorted heptagon-containing nanographenes. Chem. Sci. 2017, 8, 1068–1074.
- 11 Medel, M. A.; Cruz, C. M.; Miguel, D.; Blanco, V.; Morcillo, S. P.; Campaña, A. G. Chiral Distorted Hexa-peri-hexabenzocoronenes Bearing a Nonagon-Embedded Carbohelicene. Angew. Chem. Int. Ed. 2021, 60, 22051–22056.
- 12 Yang, L.; Ju, Y.-Y.; Medel, M. A.; Fu, Y.; Komber, H.; Dmitrieva, E.; Zhang, J.-J.; Obermann, S.; Campaña, A. G.; Ma, J.; Feng, X. Helical Bilayer Nonbenzenoid Nanographene Bearing a [10]Helicene with Two Embedded Heptagons. Angew. Chem. Int. Ed. 2023, 62, e202216193.
- 13 Birks, J. B.; Birch, D. J. S.; Cordemans, E.; Vander Donckt, E. Fluorescence of the higher helicenes. Chem. Phys. Lett. 1976, 43, 33–36.
- 14 Sapir, M.; Donckt, E. V. Intersystem crossing in the helicenes. Chem. Phys. Lett. 1975, 36, 108–110.
- 15 Oyama, H.; Akiyama, M.; Nakano, K.; Naito, M.; Nobusawa, K.; Nozaki, K. Synthesis and Properties of [7]Helicene-like Compounds Fused with a Fluorene Unit. Org. Lett. 2016, 18, 3654–3657.
- 16 Fujikawa, T.; Preda, D. V.; Segawa, Y.; Itami, K.; Scott, L. T. Corannulene–Helicene Hybrids: Chiral π-Systems Comprising Both Bowl and Helical Motifs. Org. Lett. 2016, 18, 3992–3995.
- 17 Cruz, C. M.; Castro-Fernández, S.; Maçôas, E.; Cuerva, J. M.; Campaña, A. G. Undecabenzo[7]superhelicene: A Helical Nanographene Ribbon as a Circularly Polarized Luminescence Emitter. Angew. Chem. Int. Ed. 2018, 57, 14782–14786.
- 18 Yang, Y.; Rice, B.; Shi, X.; Brandt, J. R.; Correa da Costa, R.; Hedley, G. J.; Smilgies, D.-M.; Frost, J. M.; Samuel, I. D. W.; Otero-de-la-Roza, A.; Johnson, E. R.; Jelfs, K. E.; Nelson, J.; Campbell, A. J.; Fuchter, M. J. Emergent Properties of an Organic Semiconductor Driven by Its Molecular Chirality. ACS Nano 2017, 11, 8329–8338.
- 19 Kiran, V.; Mathew, S. P.; Cohen, S. R.; Hernández Delgado, I.; Lacour, J.; Naaman, R. Helicenes—A New Class of Organic Spin Filter. Adv. Mater. 2016, 28, 1957-1962.
- 20 Ernst, K.-H. Stereochemical Recognition of Helicenes on Metal Surfaces. Acc. Chem. Res. 2016, 49, 1182–1190.
- 21 Xia, J.-X.; Li, Z.; Han, Q.; Wan, J.-J.; Shi, M.-X.; Tao, M.-L.; Sun, K.; Wang, J.-Z. Homochiral to heterochiral transition in a pentahelicene monolayer on Bi(111). Phys. Chem. Chem. Phys. 2021, 23, 24344–24348.
- 22 Irziqat, B.; Cebrat, A.; Baljozović, M.; Martin, K.; Parschau, M.; Avarvari, N.; Ernst, K. H. Stereospecific on-Surface Cyclodehydrogenation of Bishelicenes: Preservation of Handedness from Helical to Planar Chirality. Chem. Eur. J. 2021, 27, 13523–13526.
- 23 Li, J.; Martin, K.; Avarvari, N.; Wäckerlin, C.; Ernst, K.-H. Spontaneous separation of on-surface synthesized tris-helicenes into two-dimensional homochiral domains. Chem. Commun. 2018, 54, 7948–7951.
- 24 Zhong, Q.; Barát, V.; Csókás, D.; Niu, K.; Górecki, M.; Ghosh, A.; Björk, J.; Ebeling, D.; Chi, L.; Schirmeisen, A.; Stuparu Mihaiela, C. On-Surface Stereochemical Characterization of a Highly Curved Chiral Nanographene by Noncontact Atomic Force Microscopy and Scanning Tunneling Microscopy. CCS Chem. 2023, 5, 2888–2896.
- 25 Stöhr, M.; Boz, S.; Schär, M.; Nguyen, M.-T.; Pignedoli, C. A.; Passerone, D.; Schweizer, W. B.; Thilgen, C.; Jung, T. A.; Diederich, F. Self-Assembly and Two-Dimensional Spontaneous Resolution of Cyano-Functionalized [7]Helicenes on Cu(111). Angew. Chem. Int. Ed. 2011, 50, 9982–9986.
- 26 Ishii, A.; Shiotari, A.; Sugimoto, Y. Mechanically induced single-molecule helicity switching of graphene-nanoribbon-fused helicene on Au(111). Chem. Sci. 2021, 12, 13301–13306.
- 27 Kinikar, A.; Di Giovannantonio, M.; Urgel, J. I.; Eimre, K.; Qiu, Z.; Gu, Y.; Jin, E.; Narita, A.; Wang, X.-Y.; Müllen, K.; Ruffieux, P.; Pignedoli, C. A.; Fasel, R. On-surface polyarylene synthesis by cycloaromatization of isopropyl substituents. Nat. Synth. 2022, 1, 289–296.
- 28 Kato, M.; Fukui, N.; Shinokubo, H. Synthesis of Dibenzo[h,t]rubicene through Its Internally Dimethoxy-substituted Precursor. Chem. Lett. 2022, 51, 288–291.
- 29 Di Nuzzo, D.; Kulkarni, C.; Zhao, B.; Smolinsky, E.; Tassinari, F.; Meskers, S. C. J.; Naaman, R.; Meijer, E. W.; Friend, R. H. High Circular Polarization of Electroluminescence Achieved via Self-Assembly of a Light-Emitting Chiral Conjugated Polymer into Multidomain Cholesteric Films. ACS Nano 2017, 11, 12713–12722.
- 30 Watanabe, K.; Suda, K.; Akagi, K. Hierarchically self-assembled helical aromatic conjugated polymers. J. Mater. Chem. C 2013, 1, 2797–2805.
- 31 Wang, Y.; Li, Y.; Liu, S.; Li, F.; Zhu, C.; Li, S.; Cheng, Y. Regulating Circularly Polarized Luminescence Signals of Chiral Binaphthyl-Based Conjugated Polymers by Tuning Dihedral Angles of Binaphthyl Moieties. Macromolecules 2016, 49, 5444–5451.
- 32 Bartels, L.; Meyer, G.; Rieder, K. H.; Velic, D.; Knoesel, E.; Hotzel, A.; Wolf, M.; Ertl, G. Dynamics of Electron-Induced Manipulation of Individual CO Molecules on Cu(111). Phys. Rev. Lett. 1998, 80, 2004–2007.
- 33 Horcas, I.; Fernández, R.; Gómez-Rodríguez, J. M.; Colchero, J.; Gómez-Herrero, J.; Baro, A. M. WSXM: a software for scanning probe microscopy and a tool for nanotechnology. Rev. Sci. Instrum. 2007, 78, 013705.
- 34 Kresse, G.; Furthmüller, J. Efficient iterative schemes for ab initio total-energy calculations using a plane-wave basis set. Phys. Rev. B 1996, 54, 11169–11186.
- 35 Perdew, J. P.; Burke, K.; Ernzerhof, M. Generalized Gradient Approximation Made Simple. Phys. Rev. Lett. 1996, 77, 3865–3868.
- 36 Perdew, J. P.; Burke, K.; Ernzerhof, M. Generalized Gradient Approximation Made Simple. Phys. Rev. Lett. 1997, 78, 1396–1396.
- 37 Grimme, S.; Ehrlich, S.; Goerigk, L. Effect of the damping function in dispersion corrected density functional theory. J. Comput. Chem. 2011, 32, 1456–1465.




