Asymmetric Transfer Hydrogenation of Stable NH Imines for the Synthesis of Enantiopure α-Chiral Primary Amines
Mangang Zhang
College of Chemistry and Chemical Engineering, Central South University, Changsha, Hunan, 410083 China
Faculty of Pharmaceutical Sciences, Shenzhen University of Advanced Technology, Shenzhen, Guangdong, 518055 China
Search for more papers by this authorHui Li
Faculty of Pharmaceutical Sciences, Shenzhen University of Advanced Technology, Shenzhen, Guangdong, 518055 China
Search for more papers by this authorKeqin Wu
Faculty of Pharmaceutical Sciences, Shenzhen University of Advanced Technology, Shenzhen, Guangdong, 518055 China
Search for more papers by this authorNianxin Rong
Faculty of Pharmaceutical Sciences, Shenzhen University of Advanced Technology, Shenzhen, Guangdong, 518055 China
Search for more papers by this authorShaoquan Lin
Faculty of Pharmaceutical Sciences, Shenzhen University of Advanced Technology, Shenzhen, Guangdong, 518055 China
Search for more papers by this authorCorresponding Author
Hua Yang
College of Chemistry and Chemical Engineering, Central South University, Changsha, Hunan, 410083 China
E-mail: [email protected]; [email protected]Search for more papers by this authorCorresponding Author
Qin Yin
Faculty of Pharmaceutical Sciences, Shenzhen University of Advanced Technology, Shenzhen, Guangdong, 518055 China
Shenzhen Institute of Advanced Technology, Chinese Academy of Sciences, Shenzhen, Guangdong, 518055 China
E-mail: [email protected]; [email protected]Search for more papers by this authorMangang Zhang
College of Chemistry and Chemical Engineering, Central South University, Changsha, Hunan, 410083 China
Faculty of Pharmaceutical Sciences, Shenzhen University of Advanced Technology, Shenzhen, Guangdong, 518055 China
Search for more papers by this authorHui Li
Faculty of Pharmaceutical Sciences, Shenzhen University of Advanced Technology, Shenzhen, Guangdong, 518055 China
Search for more papers by this authorKeqin Wu
Faculty of Pharmaceutical Sciences, Shenzhen University of Advanced Technology, Shenzhen, Guangdong, 518055 China
Search for more papers by this authorNianxin Rong
Faculty of Pharmaceutical Sciences, Shenzhen University of Advanced Technology, Shenzhen, Guangdong, 518055 China
Search for more papers by this authorShaoquan Lin
Faculty of Pharmaceutical Sciences, Shenzhen University of Advanced Technology, Shenzhen, Guangdong, 518055 China
Search for more papers by this authorCorresponding Author
Hua Yang
College of Chemistry and Chemical Engineering, Central South University, Changsha, Hunan, 410083 China
E-mail: [email protected]; [email protected]Search for more papers by this authorCorresponding Author
Qin Yin
Faculty of Pharmaceutical Sciences, Shenzhen University of Advanced Technology, Shenzhen, Guangdong, 518055 China
Shenzhen Institute of Advanced Technology, Chinese Academy of Sciences, Shenzhen, Guangdong, 518055 China
E-mail: [email protected]; [email protected]Search for more papers by this authorComprehensive Summary
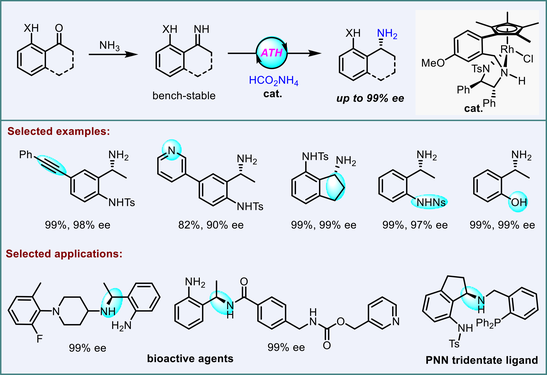
Although it offers a direct route to access synthetically valuable α-chiral primary amines, asymmetric transfer hydrogenation of NH imines has been rarely studied, due in large part to the inaccessibility and instability of NH imines. Herein, we report a Rh-catalyzed asymmetric transfer hydrogenation of a kind of novel and stable NH imines which are prepared via condensation of easily available sulfonylated 2’-aminoacetophenones with NH3 in methanol. With this method, enantioenriched chiral 2-(1-aminoalkyl)anilines, which are privileged pharmacore groups, have been synthesized with good functional group compatibility, and with up to 99% ee. A gram-scale reaction using 0.2 mol% of catalyst has been successfully performed to highlight the practicality. Furthermore, the products can be derivatized into enantiopure bioactive molecules as well as chiral tridentate ligands for metal catalysis.
Supporting Information
| Filename | Description |
|---|---|
| cjoc202400338-sup-0001-supinfo.pdfPDF document, 14.6 MB |
Appendix S1: Supporting Information |
Please note: The publisher is not responsible for the content or functionality of any supporting information supplied by the authors. Any queries (other than missing content) should be directed to the corresponding author for the article.
References
- 1(a) Li, W.; Zhang, X.-M. Stereoselective Formation of Amines, Springer, Berlin, 2014;
10.1007/978-3-642-53929-9 Google Scholar(b) Ricci, A.; Bernardi, L. Methodologies in Amine Synthesis: Challenges and Applications, Wiley-VCH, Weinheim, 2021.10.1002/9783527826186 Google Scholar
- 2(a) https://njardarson.lab.arizona.edu/content/top-pharmaceuticals-poster. (b) Nugent, T. C.; Marinova, S. M. Step-Efficient Access to Chiral Primary Amines. Synthesis 2013, 45, 153–166; (c) Yin, Q.; Shi, Y.; Wang, J.; Zhang, X. Direct catalytic asymmetric synthesis of α-chiral primary amines. Chem. Soc. Rev. 2020, 49, 6141–6153.
- 3(a) Peters, J.-U.; Lu¨bbers, T.; Alanine, A.; Kolczewski, S.; Blascob, F.; Steward, L. Cyclic guanidines as dual 5-HT5A/5-HT7 receptor ligands: Optimising brain penetration. Bioorg. Med. Chem. Lett. 2008, 18, 262–266; (b) Alanine, A.; Gobbi, L. C.; Kolczewski, S.; Luebbers, T.; Peters, J.-U.; Steward, L. (3,4-Dihydroquinazolin-2-yl)-(2-aryloxyethyl)-amines as 5-HT receptor modulators, their preparation, pharmaceutical compositions, and use in therapy. WO 2006117305-A1, 2006; (c) Sylvie, F.; Francis, H.; Mark, M.; Dorte, R.; Simon, S. C5a receptor modulators. WO 2019141808-A1, 2019; (d) Li, J.; Feng, J.; Cai, W. Histone deacetylase inhibitors and uses thereof . WO 2009152735-A1, 2009; (e) Zhu, J.; Zhang, Y.; Cao, M.; Liu, X.; Wang, Q. High stress resistant plant growth regulator and preparation method and use thereof. WO 2017114052-A1, 2017; (f) Clark, M. P.; Lyon, R. A. 6,7-Dihydro-5H-pyrazolo[1,2-a]pyrazol-1-ones which control inflammatory cytokines. US 20050148610-A1, 2005; (g) Culy, C. R.; Jarvis, B. Repaglinide: A Review of its Therapeutic Use in Type 2 Diabetes Mellitus. Drugs 2001, 61, 1625–1660; (h) Scott, L. J. Repaglinide: A Review of Its Use in Type 2 Diabetes Mellitus. Drugs 2012, 72, 249–272.
- 4(a) Wang, D.; Astruc, D. The Golden Age of Transfer Hydrogenation. Chem. Rev. 2015, 115, 6621–6686; (b) Wang, H.; Wen, J.; Zhang, X. Chiral Tridentate Ligands in Transition Metal-Catalyzed Asymmetric Hydrogenation. Chem. Rev. 2021, 121, 7530–7567; (c) Yang, F.; Xie, J.-H.; Zhou, Q.-L. Highly Efficient Asymmetric Hydrogenation Catalyzed by Iridium Complexes with Tridentate Chiral Spiro Aminophosphine Ligands. Acc. Chem. Res. 2023, 56, 332–349.
- 5(a) Martjuga, M.; Belyakov, S.; Liepinsh, E.; Suna, E. Asymmetric Synthesis of 1,3-Diamines. II: Diastereoselective Reduction of Atropisomeric N-tert-Butanesulfinylketimines. J. Org. Chem. 2011, 76, 2635–2647; (b) Achuenu, C.; Carret, S.; Poisson, J.-F.; Berthiol F. Application of Chiral Sulfinamides into Formation and Reduction of Sulfinylketimines to Obtain Valuable α-Chiral Primary Amines. Eur. J. Org. Chem. 2020, 2020, 5901–5916.
- 6For recent books on asymmetric hydrogenation, see: (a) Ratovelomanana-Vidal, V.; Phansavath, P. Asymmetric Hydrogenation and Transfer Hydrogenation, Wiley-VCH, Weinheim, 2021;
10.1002/9783527822294 Google Scholar(b) Biosca, M.; Diéguez, M.; Zanotti-Gerosa, A. Asymmetric Hydrogenation in Industry, Elsevier, Cambridge, 2021.10.1016/bs.acat.2021.08.005 Google ScholarFor selected recent reviews on the synthesis of chiral amines via asymmetric hydrogenation, see: (c) Xie, J.-H.; Zhu, S.-F.; Zhou, Q.-L. Transition Metal-Catalyzed Enantioselective Hydrogenation of Enamines and Imines. Chem. Rev. 2011, 111, 1713–1760; (d) Zhang, Z.; Butt, N. A.; Zhang, W. Asymmetric Hydrogenation of Nonaromatic Cyclic Substrates. Chem Rev. 2016, 116, 14769–14827; (e) Barrios-Rivera, J.; Xu, Y.; Wills, M.; Vyas, V. K. A diversity of recently reported methodology for asymmetric imine reduction. Org. Chem. Front. 2020, 7, 3312–3342; (f) Ponra, S.; Boudet, B.; Phansavath, P.; Ratovelomanana-Vidal, V. Recent Developments in Transition-Metal-Catalyzed Asymmetric Hydrogenation of Enamides. Synthesis 2021, 53, 193–214; (g) Tian, Y.; Hu, L.; Wang, Y.-Z.; Zhang, X.; Yin, Q. Recent advances on transition-metal-catalysed asymmetric reductive amination. Org. Chem. Front. 2021, 8, 2328–2342; (h) Abdine, R. A. A.; Hedouin, G.; Colobert, F.; Wencel-Delord, J. Metal-Catalyzed Asymmetric Hydrogenation of C=N Bonds. ACS Catal. 2021, 11, 215–247; (i) Cabré, A.; Verdaguer, X.; Riera, A. Recent Advances in the Enantioselective Synthesis of Chiral Amines via Transition Metal-Catalyzed Asymmetric Hydrogenation. Chem. Rev. 2022, 122, 269–339; (j) Liu, C.; Liu, Q. Earth-Abundant Metal-Catalyzed Asymmetric Hydrogenation of Carbon-Nitrogen Unsaturated Bonds. Chin. J. Org. Chem. 2022, 42, 3213–3220.
- 7(a) Morisaki, K.; Morimot, H.; Ohshima, T. Recent Progress on Catalytic Addition Reactions to N-Unsubstituted Imines. ACS Catal. 2020, 10, 6924–6951; (b) Shi, Y.; Rong, N.; Zhang, X.; Yin, Q. Synthesis of Chiral Primary Amines via Enantioselective Reductive Amination: From Academia to Industry. Synthesis 2023, 55, 1053–1068.
- 8(a) Hou, G.; Gosselin, F.; Li, W.; McWilliams, J. C.; Sun, Y.; Weisel, M.; O'Shea, P. D.; Chen, C.-Y.; Davies, I. W.; Zhang, X. Enantioselective Hydrogenation of N-H Imines. J. Am. Chem. Soc. 2009, 131, 9882–9883; (b) Hou, G.; Tao, R.; Sun, Y.; Zhang, X.; Gosselin, F. Iridium- Monodentate Phosphoramidite-Catalyzed Asymmetric Hydrogenation of Substituted Benzophenone N-H Imines. J. Am. Chem. Soc. 2010, 132, 2124–2125; (c) Zhao, Q.; Wen, J.; Tan, R.; Huang, K.; Metola, P.; Wang, R.; Anslyn, E. V.; Zhang, X. Rhodium-Catalyzed Asymmetric Hydrogenation of Unprotected NH Imines Assisted by a Thiourea. Angew. Chem. Int. Ed. 2014, 53, 8467–8470.
- 9 Uematsu, N.; Fujii, A.; Hashiguchi, S.; Ikariya, T.; Noyori, R. Asymmetric Transfer Hydrogenation of Imines. J. Am. Chem. Soc. 1996, 118, 4916–4917.
- 10For selected recent reviews: (a) Ikariya, T.; Blacker, A. J. Asymmetric Transfer Hydrogenation of Ketones with Bifunctional Transition Metal-Based Molecular Catalysts. Acc. Chem. Res. 2007, 40, 1300–1308; (b) Ritleng, V.; de Vries, J. G. Ruthenacycles and Iridacycles as Transfer Hydrogenation Catalysts. Molecules 2021, 26, 4076; (c) Mishra, A. A.; Bhanage, B. M. Ru-TsDPEN catalysts and derivatives in asymmetric transfer hydrogenation reactions. Chirality 2021, 33, 337–378. For selected recent examples: (d) Steward, K. M.; Gentry, E. C.; Johnson, J. S. Dynamic Kinetic Resolution of α-Keto Esters via Asymmetric Transfer Hydrogenation. J. Am. Chem. Soc. 2012, 134, 7329–7332; (e) Steward, K. M.; Corbett, M. T.; Goodman, C. G.; Johnson, J. S. Asymmetric Synthesis of Diverse Glycolic Acid Scaffolds via Dynamic Kinetic Resolution of α-Keto Esters. J. Am. Chem. Soc. 2012, 134, 20197–20206; (f) Cotman, A. E.; Cahard, D.; Mohar, B. Stereoarrayed CF3-Substituted 1,3-Diols by Dynamic Kinetic Resolution: Ruthenium(II)-Catalyzed Asymmetric Transfer Hydrogenation. Angew. Chem. Int. Ed. 2016, 55, 5294–5298; (g) Touge, T.; Sakaguchi, K.; Tamaki, N.; Nara, H.; Yokozawa, T.; Matsumura, K.; Kayaki, Y. Multiple Absolute Stereocontrol in Cascade Lactone Formation via Dynamic Kinetic Resolution Driven by the Asymmetric Transfer Hydrogenation of Keto Acids with Oxo-Tethered Ruthenium Catalysts. J. Am. Chem. Soc. 2019, 141, 16354–16361; (h) Wang, F.; Yang, T.; Wu, T.; Zheng, L.-S.; Yin, C.; Shi, Y.; Ye, X.-Y.; Chen, G.-Q., Zhang, X. Asymmetric Transfer Hydrogenation of α-Substituted-β-Keto Carbonitriles via Dynamic Kinetic Resolution. J. Am. Chem. Soc. 2021, 143, 2477–2483; (i) Ding, Y.-X.; Zhu, Z.-H.; Wang, H.; Yu, C.-B.; Zhou, Y.-G. Construction of three stereocenters via hydrogenative desymmetrization of 2,2,5-trisubstituted cyclohexane-1,3-diones. Sci. China Chem. 2021, 64, 232–237; (j) Wang, F.; Zhang, Z.; Chen, Y.; Ratovelomanana-Vidal, V.; Yu, P.; Chen, G.-Q.; Zhang, X. Stereodivergent synthesis of chiral succinimides via Rh-catalyzed asymmetric transfer hydrogenation. Nat. Commun. 2022, 13, 7794; (k) Wu, J.; Chen, Z.; Barnard, J. H.; Gunasekar, R.; Pu, C.; Wu, X.; Zhang, S.; Ruan, J.; Xiao, J. Synthesis of chiral piperidines from pyridinium salts via rhodium-catalysed transfer hydrogenation. Nat. Catal. 2022, 5, 982–992; (l) Wang, T.; Chen, J.; Wang, L.; Wang, Z.; Fan, B. Asymmetric Transfer Hydrogenation of α-Aryl Amidates Using Methanol as Hydrogen Source. Chin. J. Org. Chem. 2022, 42, 3693–3703; (m) Sterle, M.; Huš, M.; Lozinšek, M.; Zega, A.; Cotman, A. E. Hydrogen-Bonding Ability of Noyori−Ikariya Catalysts Enables Stereoselective Access to CF3-Substituted syn-1,2-Diols via Dynamic Kinetic Resolution. ACS Catal. 2023, 13, 6242–6248; (n) Chen, T.; Liu, W.; Gu, W.; Niu, S.; Lan, S.; Zhao, Z.; Gong, F.; Liu, J.; Yang, S.; Cotman, A. E.; Song, J.; Fang, X. Dynamic Kinetic Resolution of β-Substituted α-Diketones via Asymmetric Transfer Hydrogenation. J. Am. Chem. Soc. 2023, 145, 585–599; (o) Xu, L.; Yang, T.; Sun, H.; Zeng, J.; Mu, S.; Zhang, X.; Chen, G.-Q. Rhodium-Catalyzed Asymmetric Hydrogenation and Transfer Hydrogenation of 1,3-Dipolar Nitrones. Angew. Chem. Int. Ed. 2024, 63, e202319662; (p) Lan, S.; Huang, H.; Liu, W.; Xu, C.; Lei, X.; Dong, W.; Liu, J.; Yang, S.; Cotman, A. E.; Zhang, Q.; Fang, X. Asymmetric Transfer Hydrogenation of Cyclobutenediones. J. Am. Chem. Soc. 2024, 146, 4942–4957.
- 11 Mangion, I. K.; Chen, C.-Y.; Li, H.; Maligres, P.; Chen, Y.; Christensen, M.; Cohen, R.; Jeon, I.; Klapars, A.; Krska, S.; Nguyen, H.; Reamer, R. A.; Sherry, B. D.; Zavialov, I. Enantioselective Synthesis of an HCV NS5a Antagonist. Org. Lett. 2014, 16, 2310–2313.
- 12 Zheng, Y.; Clarkson, G. J.; Wills, M. Asymmetric Transfer Hydrogenation of o-Hydroxyphenyl Ketones: Utilizing Directing Effects That Optimize the Asymmetric Synthesis of Challenging Alcohols. Org. Lett. 2020, 22, 3717–3721.
- 13(a) Dai, Z.; Zhang, X.; Yin, Q. Advances on Asymmetric Reductive Amination with Ammonium Salts as Amine Sources. Chin. J. Org. Chem. 2022, 42, 2261–2274; (b) Tan, X.; Gao, S.; Zeng, W.; Xin, S.; Yin, Q.; Zhang, X. Asymmetric Synthesis of Chiral Primary Amines by Ruthenium-Catalyzed Direct Reductive Amination of Alkyl Aryl Ketones with Ammonium Salts and Molecular H2. J. Am. Chem. Soc. 2018, 140, 2024–2027; (c) Lou, Y.; Hu, Y.; Lu, J.; Guan, F.; Gong, G.; Yin, Q.; Zhang, X. Dynamic Kinetic Asymmetric Reductive Amination: Synthesis of Chiral Primary β-Amino Lactams. Angew. Chem. Int. Ed. 2018, 57, 14193–14197; (d) Hu, L.; Zhang, Y.; Zhang, Q.-W.; Yin, Q.; Zhang, X. Ruthenium-Catalyzed Direct Asymmetric Reductive Amination of Diaryl and Sterically Hindered Ketones with Ammonium Salts and H2. Angew. Chem. Int. Ed. 2020, 59, 5321–5325; (e) Shi, Y.; Tan, X.; Gao, S.; Zhang, Y.; Wang, J.; Zhang, X.; Yin, Q. Direct Synthesis of Chiral NH Lactams via Ru-Catalyzed Asymmetric Reductive Amination/Cyclization Cascade of Keto Acids/Esters. Org. Lett. 2020, 22, 2707–2713; (f) Hu, L.; Wang, Y.-Z.; Xu, L.; Yin, Q.; Zhang, X. Highly Enantioselective Synthesis of N-Unprotected Unnatural α-Amino Acid Derivatives by Ruthenium-Catalyzed Direct Asymmetric Reductive Amination. Angew. Chem. Int. Ed. 2022, 61, e202202552; (g) Shi, Y.; Wang, J.; Yang, F.; Wang, C.; Zhang X.; Chiu, P.; Yin, Q. Direct asymmetric reductive amination of α-keto acetals: a platform for synthesizing diverse α-functionalized amines. Chem. Commun. 2022, 58, 513–516.
- 14(a) Echeverria, P. G.; Zheng, L. S.; Llopis, Q.; He, B.; Westermeyer, A.; Betancourt, R. M.; Phansavath, P.; Ratovelomanana-Vidal, V. Tethered Rh(III)-N-(p-Tolylsulfonyl)-1,2-Diphenylethylene-1,2-Diamine Complexes: Efficient Catalysts for Asymmetric Transfer Hydrogenation. SynOpen 2022, 6, 75–79; (b) Zheng, L.-S.; Férard, C.; Phansavath, P.; Ratovelomanana-Vidal, V. Rhodium-mediated asymmetric transfer hydrogenation: a diastereo- and enantioselective synthesis of syn-α-amido β-hydroxy esters. Chem. Commun. 2018, 54, 283–286; (c) He, B.; Phansavath, P.; Ratovelomanana-Vidal, V. Rh-Mediated Asymmetric-Transfer Hydrogenation of 3-Substituted Chromones: A Route to Enantioenriched cis-3-(Hydroxymethyl)chroman-4-ol Derivatives through Dynamic Kinetic Resolution. Org. Lett. 2019, 21, 3276–3280; (d) Westermeyer, A.; Guillamot, G.; Phansavath, P.; Ratovelomanana-Vidal, V. Synthesis of Enantioenriched β-Hydroxy-γ- Acetal Enamides by Rhodium-Catalyzed Asymmetric Transfer Hydrogenation. Org. Lett. 2020, 22, 3911–3914; (e) Betancourt, R. M.; Phansavath, P.; Ratovelomanana-Vidal, V. Rhodium-Catalyzed Asymmetric Transfer Hydrogenation/Dynamic Kinetic Resolution of 3-Benzylidene-Chromanones. Org. Lett. 2021, 23, 1621–1625. For the first TsDpen-tethered Rh complex for ATH reactions, see: (f) Matharu, D. S.; Morris, D. J.; Kawamoto, A. M.; Clarkson, G. J.; Wills, M. A Stereochemically Well-Defined Rhodium(III) Catalyst for Asymmetric Transfer Hydrogenation of Ketones. Org. Lett. 2005, 7, 5489–5491; (g) Matharu, D. S.; Martins, J. E. D.; Wills, M. Asymmetric Transfer Hydrogenation of C=O and C=N Bonds by Tethered Rh(III) Catalysts. Chem. Asian J. 2008, 3, 1374–1383.
- 15For selected examples, see: (a) Hayes, A. M.; Morris, D. J.; Clarkson, G. J.; Wills, M. A Class of Ruthenium(II) Catalyst for Asymmetric Transfer Hydrogenations of Ketones. J. Am. Chem. Soc. 2005, 127, 7318–7319; (b) Vyas, V. K.; Clarkson, G. J.; Wills, M. Sulfone Group as a Versatile and Removable Directing Group for Asymmetric Transfer Hydrogenation of Ketones. Angew. Chem. Int. Ed. 2020, 59, 14265–14269; (c) Zheng, Y.; Martinez-Acosta, J. A.; Khimji, M.; Barbosa, L. C. A.; Clarkson, G. J.; Wills, M. Asymmetric Transfer Hydrogenation of Aryl Heteroaryl Ketones using Noyori-Ikariya Catalysts. ChemCatChem 2021, 13, 4384–4391; (d) Gediya, S. K.; Vyas, V. K.; Clarkson, G. J.; Wills, M. Asymmetric Transfer Hydrogenation of α-Keto Amides; Highly Enantioselective Formation of Malic Acid Diamides and α-Hydroxyamides. Org. Lett. 2021, 23, 7803–7807.
- 16(a) Touge, T.; Hakamata, T.; Nara, H.; Kobayashi, T.; Sayo, N.; Saito, T.; Kayaki, Y.; Ikariya, T. Oxo-Tethered Ruthenium(II) Complex as a Bifunctional Catalyst for Asymmetric Transfer Hydrogenation and H2 Hydrogenation. J. Am. Chem. Soc. 2011, 133, 14960–14963; (b) Parekh, V.; Ramsdenb, J. A.; Wills, M. Ether-tethered Ru(II)/TsDPEN complexes; synthesis and applications to asymmetric transfer hydrogenation. Catal. Sci. Technol. 2012, 2, 406–414.
- 17 Wang, L.; Lin, J.; Xia, C.; Sun, W. Iridium-Catalyzed Asymmetric Transfer Hydrogenation of Quinolines in Biphasic Systems or Water. J. Org. Chem. 2021, 86, 16641−16651.
- 18(a) Rostovtsev, V. V.; Green, L. G.; Fokin, V. V.; Sharpless, K. B. A Stepwise Huisgen Cycloaddition Process: Copper(I)-Catalyzed Regioselective “Ligation” of Azides and Terminal Alkynes. Angew. Chem. Int. Ed. 2002, 41, 2596–2599;
10.1002/1521-3773(20020715)41:14<2596::AID-ANIE2596>3.0.CO;2-4 CAS PubMed Web of Science® Google Scholar(b) Goddard-Borger, E. D.; Stick, R. V. An Efficient, Inexpensive, and Shelf-Stable Diazotransfer Reagent: Imidazole-1-sulfonyl Azide Hydrochloride. Org. Lett. 2007, 9, 3797–3800; (c) Meng, G.; Guo, T.; Ma, T.; Zhang, J.; Shen, Y.; Sharpless, K. B.; Dong, J. Modular click chemistry libraries for functional screens using a diazotizing reagent. Nature 2019, 574, 86–89.
- 19 Niu, T.; Liu, L. X.; Wu, B.; Zhou Y. G. Synthesis of Tridentate PNO Ligands with Planar Chirality and Application in Iridium-Catalyzed Asymmetric Hydrogenation of Simple Ketones. J. Org. Chem. 2023, 88, 7863–7871.




