Palladium-Catalyzed Selective Syntheses of 2,3-Allenyl Amines via Double Functionalization Coupling of 2-Alkynyl-1,4-diol Dicarbonates
Zhengnan Zhou
State Key Laboratory of Organometallic Chemistry, Shanghai Institute of Organic Chemistry, Chinese Academy of Sciences, 345 Lingling Lu, Shanghai, 200032 China
University of Chinese Academy of Sciences, Beijing, 100049 China
Search for more papers by this authorCan Li
State Key Laboratory of Organometallic Chemistry, Shanghai Institute of Organic Chemistry, Chinese Academy of Sciences, 345 Lingling Lu, Shanghai, 200032 China
University of Chinese Academy of Sciences, Beijing, 100049 China
Search for more papers by this authorCorresponding Author
Shengming Ma
State Key Laboratory of Organometallic Chemistry, Shanghai Institute of Organic Chemistry, Chinese Academy of Sciences, 345 Lingling Lu, Shanghai, 200032 China
Research Center for Molecular Recognition and Synthesis, Department of Chemistry, Fudan University, 220 Handan Lu, Shanghai, 200433 China
E-mail: [email protected]Search for more papers by this authorZhengnan Zhou
State Key Laboratory of Organometallic Chemistry, Shanghai Institute of Organic Chemistry, Chinese Academy of Sciences, 345 Lingling Lu, Shanghai, 200032 China
University of Chinese Academy of Sciences, Beijing, 100049 China
Search for more papers by this authorCan Li
State Key Laboratory of Organometallic Chemistry, Shanghai Institute of Organic Chemistry, Chinese Academy of Sciences, 345 Lingling Lu, Shanghai, 200032 China
University of Chinese Academy of Sciences, Beijing, 100049 China
Search for more papers by this authorCorresponding Author
Shengming Ma
State Key Laboratory of Organometallic Chemistry, Shanghai Institute of Organic Chemistry, Chinese Academy of Sciences, 345 Lingling Lu, Shanghai, 200032 China
Research Center for Molecular Recognition and Synthesis, Department of Chemistry, Fudan University, 220 Handan Lu, Shanghai, 200433 China
E-mail: [email protected]Search for more papers by this authorComprehensive Summary
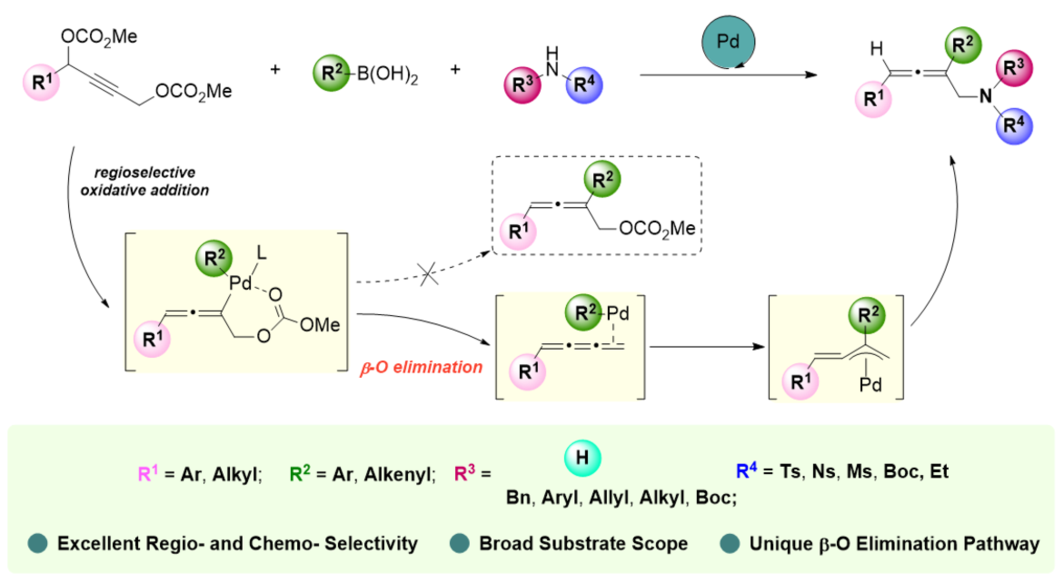
2,3-Allenyl amines have shown wide applicability in biomedical and synthetic applications. Due to their enormous potential for applications, researchers have been dedicated to the development of methods for synthesizing 2,3-allenyl amines. Herein, a palladium-catalyzed three-component reaction of 2-alkynyl-1,4-diol dicarbonates, organoboronic acids, and nitrogen nucleophiles forming 2,3-allenyl amines with excellent regio- and chemo-selectivity has been developed. Substrate compatibility and synthetic applications have been demonstrated. Control experiments supported a mechanism involving 1,2,3-triene-Pd species and methylene-π-allyl palladium species.
Supporting Information
| Filename | Description |
|---|---|
| cjoc202400298-sup-0001-Supinfo.pdfPDF document, 12.3 MB |
Appendix S1: Supporting Information |
Please note: The publisher is not responsible for the content or functionality of any supporting information supplied by the authors. Any queries (other than missing content) should be directed to the corresponding author for the article.
References
- 1For selected reviews on synthetic applications of 2,3-allenyl amines: (a) Alcaide, B.; Almendros, P. Novel cyclization reactions of aminoallenes. Adv. Synth. Catal. 2011, 353, 2561–2576; (b) Muñoz, M. P. Silver and platinum-catalysed addition of O-H and N-H bonds to allenes. Chem. Soc. Rev. 2014, 43, 3164–3183; (c) Ye, J.; Ma, S. Palladium-catalyzed cyclization reactions of allenes in the presence of unsaturated carbon–carbon bonds. Acc. Chem. Res. 2014, 47, 989–1000.
- 2For selected reports on applications of 2,3-allenyl amines: (a) Halliday, R. P.; Davis, C. S.; Heotis, J. P.; Pals, D. T.; Watson, E. J.; Bickerton, R. K. Allenic amines: a new class of nonhydrazine MAO inhibitors. J. Pharm. Sci. 1968, 57, 430–433; (b) Sahlberg, C.; Ross, S. B.; Fagervall, I.; Ask, A. L.; Claesson, A. Synthesis and monoamine oxidase inhibitory activities of α-allenic amines in vivo and in vitro. Different activities of two enantiomeric allenes. J. Med. Chem. 1983, 26, 1036–1042; (c) Castelhano, A. L.; Pliura, D. H.; Taylor, G. J.; Hsieh, K. C.; Krantz, A. Allenic suicide substrates. New inhibitors of vitamin B6 linked decarboxylases. J. Am. Chem. Soc. 1984, 106, 2734–2735; (d) Sarhan, S.; Casara, P.; Knödgen, B.; Seiler, N. (4S)-4-amino-5,6-heptadienoic acid (MDL 72483): A potent anticonvulsant GABA-T inhibitor. Neurochem. Res. 1991, 16, 285–293; (e) Ohno, H.; Toda, A.; Miwa, Y.; Taga, T.; Osawa, E.; Yamaoka, Y.; Fujii, N.; Ibuka, T. First palladium-catalyzed aziridination reaction of amino allenes. J. Org. Chem. 1999, 64, 2992–2993; (f) Shu, W.; Yu, Q.; Jia, G.; Ma, S. Palladium-catalyzed three-component reaction of 2,3-allenyl amines, isocyanates, and organic halides: A diversified assembly of imidazolidinones. Chem. Eur. J. 2011, 17, 4720–4723; (g) Cheng, J.; Tang, X.; Yu, Y.; Ma, S. FeCl3-catalyzed cyclization of α-sulfonamido-allenes with aldehydes—the substituent effect. Chem. Commun. 2012, 48, 12074–12076; (h) Li, S.; Ye, J.; Yuan, W.; Ma, S. Highly regioselective three-component palladium-catalyzed synthesis of 5-vinyloxazolidin- 2-ones from 2,3-allenyl amines, organic iodides, and carbon dioxide. Tetrahedron 2013, 69, 10450–10456; (i) Luo, H.; Yang, Z.; Lin, W.; Zheng, Y.; Ma, S. A catalytic highly enantioselective allene approach to oxazolines. Chem. Sci. 2018, 9, 1964–1969; (j) Han, Y.; Qin, A.; Zhang, Q.; Zhang, X.; Qian, H.; Ma, S. Rhodium-catalyzed dynamic kinetic [4+2] cycloaddition of allene-1,3-dienes. Angew. Chem. Int. Ed. 2022, 61, e202211635.
- 3(a) Radhakrishnan, U.; Al-Masum, M.; Yamamoto, Y. Palladium catalyzed hydroamination of conjugated enynes. Tetrahedron Lett. 1998, 39, 1037–1040; (b) Brinkmann, C.; Barrett, A. G. M.; Hill, M. S.; Procopiou, P. A. Heavier alkaline earth catalysts for the intermolecular hydroamination of vinylarenes, dienes, and alkynes. J. Am. Chem. Soc. 2012, 134, 2193–2207; (c) Adamson, N. J.; Jeddi, H.; Malcolmson, S. J. Preparation of chiral allenes through Pd-catalyzed intermolecular hydroamination of conjugated enynes: Enantioselective synthesis enabled by catalyst design. J. Am. Chem. Soc. 2019, 141, 8574–8583; (d) Zhang, Y.; Yu, B.; Gao, B.; Zhang, T.; Huang, H. Triple-bond insertion triggers highly regioselective 1,4-aminomethylamination of 1,3-enynes with aminals enabled by Pd-catalyzed C-N bond activation, Org. Lett. 2019, 21, 535–539; (e) Zou, S.; Yu, B.; Huang, H. Palladium-catalyzed ring-closing aminoalkylative amination of unactivated aminoenynes. Angew. Chem. Int. Ed. 2023, 62, e202215325.
- 4(a) Ogasawara, M.; Ikeda, H.; Hayashi, T. π-Allylpalladium-Mediated Catalytic Synthesis of Functionalized Allenes. Angew. Chem. Int. Ed. 2000, 39, 1042–1044;
10.1002/(SICI)1521-3773(20000317)39:6<1042::AID-ANIE1042>3.0.CO;2-7 CAS PubMed Web of Science® Google Scholar(b) Ogasawara, M.; Ueyama, K.; Nagano, T.; Mizuhata, Y.; Hayashi, T. Palladium-catalyzed asymmetric synthesis of axially chiral (allenylmethyl)silanes and chirality transfer to stereogenic carbon centers in SE’ reactions. Org. Lett. 2003, 5, 217–219; (c) Ogasawara, M.; Ge, Y.; Uetake, K.; Takahashi, T. Vinyl ketones to allenes: Preparation of 1,3-dien-2-yl triflates and their application in Pd-catalyzed reactions with soft nucleophiles. Org. Lett. 2005, 7, 5697–5700; (d) Ogasawara, M.; Okada, A.; Nakajima, K.; Takahashi, T. Palladium-catalyzed synthesis of endocyclic allenes and their application in stereoselective [2 + 2] cycloaddition with ketenes. Org. Lett. 2009, 11, 177–180.
- 5(a) Imada, Y.; Nishida, M.; Kutsuwa, K.; Murahashi, S.-I.; Naota, T. Palladium-catalyzed asymmetric amination and imidation of 2,3-allenyl phosphates. Org. Lett. 2005, 7, 5837–5839; (b) Trost, B. M.; Fandrick, D. R.; Dinh, D. C. Dynamic kinetic asymmetric allylic alkylations of allenes. J. Am. Chem. Soc. 2005, 127, 14186–14187; (c) Imada, Y.; Nishida, M.; Naota, T. Sequential asymmetric homoallenylation of primary amines with a palladium catalyst. Tetrahedron Lett. 2008, 49, 4915–4917; (d) Boutier, A.; Kammerer-Pentier, C.; Krause, N.; Prestat, G.; Poli, G. Pd-catalyzed asymmetric synthesis of N-allenyl amides and their Au-catalyzed cycloisomerizative hydroalkylation: A new route toward enantioenriched pyrrolidones. Chem. Eur. J. 2012, 18, 3840–3844; (e) Wan, B.; Ma, S. Enantioselective decarboxylative amination: Synthesis of axially chiral allenyl amines. Angew. Chem. Int. Ed. 2013, 52, 441–445; (f) Li, Q.; Fu, C.; Ma, S. Palladium-catalyzed asymmetric amination of allenyl phosphates: Enantioselective synthesis of allenes with an additional unsaturated unit. Angew. Chem. Int. Ed. 2014, 53, 6511–6514; (g) Glatz, F.; Petrone, D. A.; Carreira, E. M. Ir-catalyzed enantioconvergent synthesis of diversely protected allenylic amines employing ammonia surrogates. Angew. Chem. Int. Ed. 2020, 59, 16404–16408; (h) Cui, Y.; Zhai, Y.; Xiao, J.; Li, C.; Zheng, W.-F.; Huang, C.; Wu, G.; Qin, A.; Lin, J.; Liu, Q.; Wang, H.; Wu, P.; Xu, H.; Zheng, Y.; Ma, S. Chirality memory of α-methylene-π-allyl iridium species. Chem. Sci. 2021, 12, 11831–11838; (i) Xiao, X.; Lu, Y.-J.; Tian, H.-Y.; Zhou, H.-J.; Li, J.-W.; Yao, Y.-P.; Ke, M.-L.; Chen, F.-E. Organocatalytic atroposelective N-alkylation: divergent synthesis of axially chiral sulfonamides and biaryl amino phenols. Org. Chem. Front. 2022, 9, 2830–2839; (j) Zha, T.; Rui, J.; Zhang, Z.; Zhang, D.; Yang, Z.; Yu, P.; Wang, Y.; Peng, F.; Shao, Z. Direct catalytic asymmetric and regiodivergent N1- and C3-allenylic alkylation of indoles. Angew. Chem. Int. Ed. 2023, 62, e202300844.
- 6 Li, C.; Zhou, Z.; Ma, S. A Pd-catalyzed highly selective three-component protocol for trisubstituted allenes. Chem. Sci. 2023, 14, 7709–7715.
- 7 Dieter, R. K.; Yu, H. Synthesis of 3-Pyrrolines, Annulated 3-Pyrrolines, and Pyrroles from α-Amino Allenes. Org. Lett. 2001, 3, 3855–3858.
- 8(a) Mitsunobu, O.; Yamada, M. Preparation of esters of carboxylic and phosphoric acid via quaternary phosphonium salts. Bull. Chem. Soc. Jpn. 1967, 40, 2380–2382; (b) Han, Y.; Zhao, Y.; Ma, S. Rhodium-catalyzed Pauson-Khand-Type cyclization of 1,5-allene-alkynes: A chirality transfer strategy for optically active bicyclic ketones. Chem. Eur. J. 2019, 25, 9529–9533.
- 9 Huck, J.; Duru, C.; Roumestant, M. L.; Martinez, J. Parallel synthesis of new β2-amino esters via conjugate nucleophilic additions. Synthesis 2003, 14, 2165–2168.
- 10 Lyu, H.; Kevlishvili, I.; Yu, X.; Liu, P.; Dong, G. Boron insertion into alkyl ether bonds via zinc/nickel tandem catalysis. Science 2021, 372, 175–182.




