Reduction of Tertiary Carbon Radicals via Asymmetric Hydrogen Atom Transfer (AHAT)†
Xue Han
Shenzhen Grubbs Institute and Department of Chemistry, Guangdong Provincial Key Laboratory of Catalysis, Southern University of Science and Technology, Shenzhen, Guangdong, 518055 China
Search for more papers by this authorCorresponding Author
Chuan He
Shenzhen Grubbs Institute and Department of Chemistry, Guangdong Provincial Key Laboratory of Catalysis, Southern University of Science and Technology, Shenzhen, Guangdong, 518055 China
E-mail: [email protected]Search for more papers by this authorXue Han
Shenzhen Grubbs Institute and Department of Chemistry, Guangdong Provincial Key Laboratory of Catalysis, Southern University of Science and Technology, Shenzhen, Guangdong, 518055 China
Search for more papers by this authorCorresponding Author
Chuan He
Shenzhen Grubbs Institute and Department of Chemistry, Guangdong Provincial Key Laboratory of Catalysis, Southern University of Science and Technology, Shenzhen, Guangdong, 518055 China
E-mail: [email protected]Search for more papers by this author† Dedicated to the Special Issue of Emerging Investigators in 2024.
Abstract
Comprehensive Summary
In the past two decades, the development of asymmetric radical reactions has achieved tremendous progress, which has emerged as a powerful tool for the synthesis of chiral molecules in synthetic chemistry. Among the diverse array of radical processes, the transfer of hydrogen atoms to tertiary carbon radicals offers the potential for constructing chiral tertiary carbon centers in a stereoselective fashion. Notwithstanding the challenges associated with the reactive and evanescent nature of radical species, the use of chiral reagents or mediators has enabled the stereocontrol of the asymmetric hydrogen atom transfer (AHAT), which provides novel avenues for advancing the field of asymmetric synthesis.
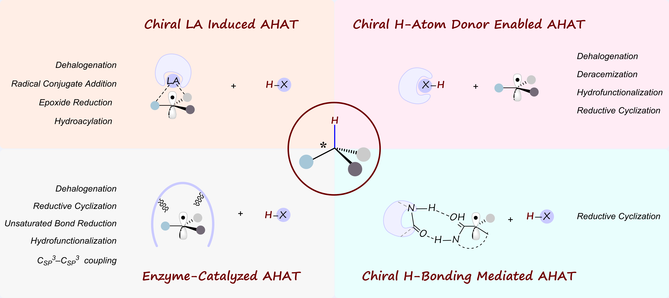
Key Scientists
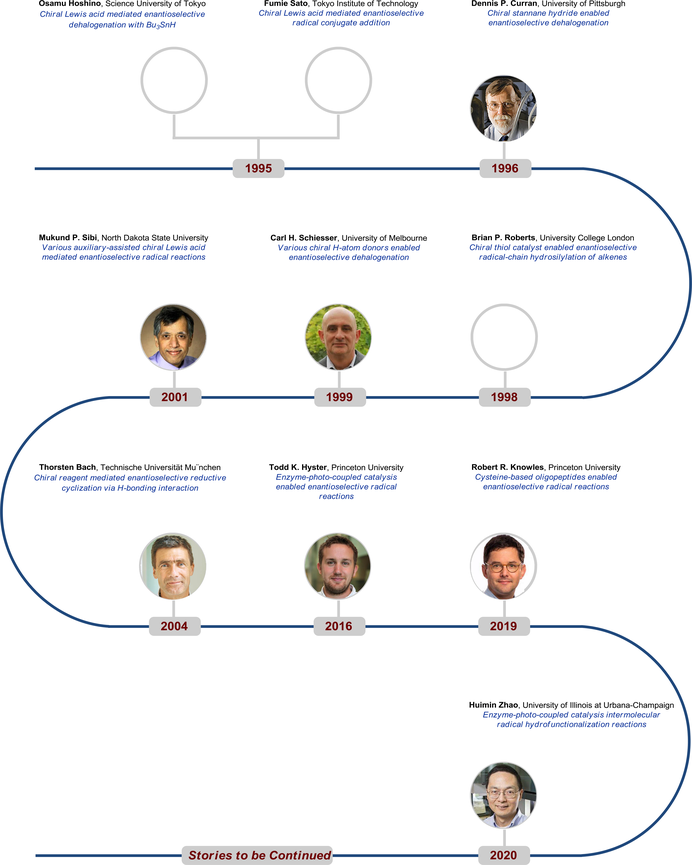
References
- 1 Yan, M.; Lo, J. C.; Edwards, J. T.; Baran, P. S. Radicals: Reactive Intermediates with Translational Potential. J. Am. Chem. Soc. 2016, 138, 12692–12714.
- 2 Smith, J. M.; Harwood, S. J.; Baran, P. S. Radical Retrosynthesis. Acc. Chem. Res. 2018, 51, 1807–1817.
- 3 Studer, A.; Curran, D. P. Catalysis of Radical Reactions: A Radical Chemistry Perspective. Angew. Chem. Int. Ed. 2016, 55, 58–102.
- 4 Sibi, M. P.; Manyem, S.; Zimmerman, J. Enantioselective Radical Processes. Chem. Rev. 2003, 103, 3263−3295.
- 5 Proctor, R. S. J.; Colgan, A. C.; Phipps, R. J. Exploiting Attractive Non- Covalent Interactions for the Enantioselective Catalysis of Reactions Involving Radical Intermediates. Nat. Chem. 2020, 12, 990−1004.
- 6 Mondal, S.; Dumur, F.; Gigmes, D.; Sibi, M. P.; Bertrand, M. P.; Nechab, M. Enantioselective Radical Reactions Using Chiral Catalysts. Chem. Rev. 2022, 122, 5842–5976.
- 7 Capaldo, L.; Ravelli, D. Hydrogen Atom Transfer (HAT): A Versatile Strategy for Substrate Activation in Photocatalyzed Organic Synthesis. Eur. J. Org. Chem. 2017, 2056−2071.
- 8 Cao, H.; Tang, X.; Tang, H.; Yuan, Y.; Wu, J. Photoinduced Intermolecular Hydrogen Atom Transfer Reactions in Organic Synthesis. Chem. Catal. 2021, 1, 523–598.
- 9 Subramanian, H.; Sibi, M. P. Stereoselective Hydrogen Atom Transfer in Free Radical Reactions. Asian J. Org. Chem. 2023, 12, e202300175.
- 10 Murakata, M.; Tsutsui, H.; Hoshino, O. Enantioselective Radical-Mediated Reduction of α-lodolactone using Tributyltin Hydride in the Presence of a Chiral Amine and a Lewis Acid. J. Chem. Soc. Chem. Commun. 1995, 481–482.
- 11 Murakata, M.; Tsutsui, H.; Takeuchi, N.; Hoshino, O. Enantioselective Radical-Mediated Reduction of α-Alkyl-α-Iododihydrocoumarins in the Presence of a Chiral Magnesium Iodide. Tetrahedron 1999, 55, 10295–10304.
- 12 Urabe, H.; Yamashita, K.; Suzuki, K.; Kobayashi, K.; Sato, F. Lewis Acid-Enhanced Reactivity of α,β-Unsaturated Ester and Amide toward Radical Addition. J. Org. Chem. 1995, 60, 3576–3577.
- 13 Sibi, M. P.; Ji, J.; Wu, J. H.; Gürtler, S.; Porter, N. A. Chiral Lewis Acid Catalysis in Radical Reactions: Enantioselective Conjugate Radical Additions. J. Am. Chem. Soc. 1996, 118, 9200–9201.
- 14 Sibi, M. P.; Sausker, J. B. The Role of the Achiral Template in Enantioselective Transformations. Radical Conjugate Additions to α-Methacrylates Followed by Hydrogen Atom Transfer. J. Am. Chem. Soc. 2002, 124, 984–991.
- 15 Sugimoto, H.; Nakamura, S.; Watanabe, Y.; Toru, T. Enantioselective Hydrogen Atom Transfer to α-Sulfonyl Radicals Controlled by Selective Coordination of a Chiral Lewis Acid to an Enantiotopic Sulfonyl Oxygen. Tetrahedron: Asymmetry 2003, 14, 3043–3055.
- 16
Sibi, M. P.; Asano, Y.; Sausker, J. B. Enantioselective Hydrogen Atom Transfer Reactions: Synthesis of N-Acyl-α-Amino Acid Esters. Angew. Chem. Int. Ed. 2001, 40, 1293–1296.
10.1002/1521-3773(20010401)40:7<1293::AID-ANIE1293>3.0.CO;2-Y CAS PubMed Web of Science® Google Scholar
- 17 Sibi, M. P.; Patil, K. Enantioselective H-Atom Transfer Reactions: A New Methodology for the Synthesis of β2-Amino Acids. Angew. Chem. Int. Ed. 2004, 43, 1235–1238.
- 18 Sibi, M. P.; Patil, K. Enantioselective H-Atom Transfer Reaction: A Strategy to Synthesize Formaldehyde Aldol Products. Org. Lett. 2005, 7, 1453–1456.
- 19 Xu, Z.; Shen, J.; Li, L.; Chen, W.; Li, S.; Jiang, J.; Zhang, Y. Q. (Salen)Titanium-Catalyzed Asymmetric Hydrogen Atom Transfer for Epoxides Reduction. Angew. Chem. Int. Ed. 2022, 61, e202214111.
- 20 Luo, Y.; Wei, Q.; Yang, L.; Zhou, Y.; Cao, W.; Su, Z.; Liu, X.; Feng, X. Enantioselective Radical Hydroacylation of α,β-Unsaturated Carbonyl Compounds with Aldehydes by Triplet Excited Anthraquinone. ACS Catal. 2022, 12, 12984–12992.
- 21 Aechtner, T.; Dressel, M.; Bach, T. Hydrogen Bond Mediated Enantioselectivity of Radical Reactions. Angew. Chem. Int. Ed. 2004, 43, 5849–5851.
- 22 Yin, Y.; Dai, Y.; Jia, H.; Li, J.; Bu, L.; Qiao, B.; Zhao, X.; Jiang, Z. Conjugate Addition-Enantioselective Protonation of N-Aryl Glycines to α-Branched 2-Vinylazaarenes via Cooperative Photoredox and Asymmetric Catalysis. J. Am. Chem. Soc. 2018, 140, 6083–6087.
- 23 Kong, M.; Tan, Y.; Zhao, X.; Qiao, B.; Tan, C.-H.; Cao, S.; Jiang, Z. Catalytic Reductive Cross Coupling and Enantioselective Protonation of Olefins to Construct Remote Stereocenters for Azaarenes. J. Am. Chem. Soc. 2021, 143, 4024–4031.
- 24 Tan, Y.; Yin, Y.; Cao, S.; Zhao, X.; Qu, G.; Jiang, Z. Conjugate Addition-Enantioselective Protonation to Forge Tertiary Stereocentres α to Azaarenes via Cooperative Hydrogen Atom Transfer and Chiral Hydrogen-Bonding Catalysis. Chin. J. Catal. 2022, 43, 558–563.
- 25 Gu, Z.; Zhang, L.; Li, H.; Cao, S.; Yin, Y.; Zhao, X.; Ban, X.; Jiang, Z. Deracemization through Sequential Photoredox-Neutral and Chiral Brønsted Acid Catalysis. Angew. Chem. Int. Ed. 2022, 61, e202211241.
- 26 Ohno, A.; Ikeguchi, M.; Kimura, T.; Oka, S. Reduction by a Model of NAD(P)H. 25. A Chiral Model Which Induces High Asymmetry. J. Am. Chem. Soc. 1979, 101, 7036–7040.
- 27 Tanner, D. D.; Kharrat, A. Free-Radical Reduction Reactions of Chiral Dihydronicotinamides. Enantioselective Hydrogen Atom Transfer and Electron-Transfer Processes During the Reduction of Ketones. J. Am. Chem. Soc. 1988, 110, 2968–2970.
- 28 Nanni, D.; Curran, D. P. Synthesis and Some Reactions of the First Chiral Tin Hydride Containing a C2-Symmetric Binaphthyl Substituent. Tetrahedron Asymmetry 1996, 7, 2417–2422.
- 29 Blumenstein, M.; Schwarzkopf, K.; Metzger, J. O. Enantioselective Hydrogen Transfer from a Chiral Tin Hydride to a Prochiral Carbon- Centered Radical. Angew. Chem. Int. Ed. 1997, 36, 235–236.
- 30 Schwarzkopf, K.; Blumenstein, M.; Hayen, A.; Metzger, J. O. Enantioselectivity of the Transfer of Hydrogen Atoms to Acyclic Prochiral Carbon-Centred Radicals Using Chiral Tin Hydrides. Eur. J. Org. Chem. 1998, 177–181.
- 31
Perchyonok, V. T.; Schiesser, C. H. Enantioselectivity Testing of Chiral Stannanes Derived from Menthol. Phosphorus, Sulfur, Silicon Relat. Elem. 1999, 150, 193–199.
10.1080/10426509908546384 Google Scholar
- 32
Skidmore, M. A.; Schiesser, C. H. Toward Chiral Stannane Reducing Agents Derived from Cholic Acid and Cholestanol. Phosphorus, Sulfur, Silicon Relat. Elem. 1999, 150, 177–191.
10.1080/10426509908546383 Google Scholar
- 33
Helliwell, M.; Thomas, E. J.; Townsend, L. A. Synthesis of Chiral Organotin Reagents: Synthesis of Enantiomerically Enriched Bicyclo[2.2.1]hept-2-yl Tin Hydrides from Camphor. X-Ray Crystal Structures of (Dimethyl)[(1R,2S,4R)-1,7,7-trimethylbicyclo[2.2.1]hept-2- yl]tin Chloride and Methyl(phenyl)bis[(1R,2S,4R)-1,7,7-trimethylbicyclo[2.2.1]hept-2-yl]stannane. J. Chem. Soc., Perkin Trans. 1 2002, 1286–1296.
10.1039/b200317c Google Scholar
- 34 Gualtieri, G.; Geib, S. J.; Curran, D. P. A New Class of Chiral Organogermanes Derived from C2-Symmetric Dithiols: Synthesis, Characterization and Stereoselective Free Radical Reactions. J. Org. Chem. 2003, 68, 5013–5019.
- 35
Curran, D. P.; Gualtieri, G. Synthesis and Asymmetric Hydrogermylation Reactions of Dithiogermanium Hydrides Derived from C2-Symmetric Dithiols. Synlett 2001, SI, 1038–1041.
10.1055/s-2001-14655 Google Scholar
- 36 Kang, J.; Kim, T. H. Asymmetric Radical Reduction with Planar Chiral Organotin Hydrides. Bull. Korean Chem. Soc. 2003, 24, 1055–1056.
- 37 Dakternieks, D.; Dunn, K.; Perchyonok, V. T.; Schiesser, C. H. Remarkable Lewis Acid Mediated Enhancement of Enantioselectivity During Free-Radical Reductions by Simple Chiral Non-Racemic Stannanes. Chem. Commun. 1999, 1665–1666.
- 38 Dakternieks, D.; Schiesser, C. H. The Quest for Single-Enantiomer Outcomes in Free-Radical Chemistry. Aust. J. Chem. 2001, 54, 89–91.
- 39 Schiesser, C. H.; Skidmore, M. A.; White, J. M. Stannanes from Cholic Acid as Enantioselective Free-Radical Reducing Agents. Aust. J. Chem. 2001, 54, 199–204.
- 40 Dakternieks, D.; Perchyonok, V. T.; Schiesser, C. H. Single Enantiomer Free-Radical Chemistry—Lewis Acid-Mediated Reductions of Racemic Halides Using Chiral Non-Racemic Stannanes. Tetrahedron: Asymmetry 2003, 14, 3057–3068.
- 41 Blumenstein, M.; Lemmler, M.; Hayen, A.; Metzger, J. O. Enantioselective Hydrogen Transfer Reactions from Chiral Binaphthyl Variants of Tin Hydrides to Prochiral Radicals. Tetrahedron: Asymmetry 2003, 14, 3069–3077.
- 42 Zeng, L.; Dakternieks, D.; Duthie, A.; Perchyonok, V. T.; Schiesser, C. H. Synthesis, Characterization and Enantioselective Free Radical Reductions of (1R,2S,5R)-Menthyldiphenylgermane and its Enantiomer. Tetrahedron: Asymmetry 2004, 15, 2547–2554.
- 43 Rettenmeier, C.; Wadepohl, H.; Gade, L. H. Stereoselective Hydrodehalogenation via a Radical-Based Mechanism Involving T-Shaped Chiral Nickel(I) Pincer Complexes. Chem. Eur. J. 2014, 20, 9657–9665.
- 44 Shin, N. Y.; Ryss, J. M.; Zhang, X.; Miller, S. J.; Knowles, R. R. Light- Driven Deracemization Enabled by Excited-State Electron Transfer. Science 2019, 366, 364–369.
- 45 Dang, H.-S.; Roberts, B. P. Enantioselective Hydrogen-atom Abstraction by Homochiral Silanethiyl Radicals. Tetrahedron Lett. 1995, 36, 3731–3734.
- 46 Haque, M. B.; Roberts, B. P. Enantioselective Radical-Chain Hydrosilylation of Prochiral Alkenes Using Optically Active Thiol Catalysts. Tetrahedron Lett. 1996, 37, 9123–9126.
- 47
Haque, M. B.; Roberts, B. P.; Tocher, D. A. Enantioselective Radical- Chain Hydrosilylation of Alkenes Using Homochiral Thiols as Polarity- Reversal Catalysts. J. Chem. Soc., Perkin Trans. 1 1998, 2881–2889.
10.1039/a803280g Google Scholar
- 48 Dang, H.-S.; Kim, K.-M.; Roberts, B. P. Radical-Chain Reductive Carboxyalkylation of Electron-Rich Alkenes: Carbon–Carbon Bond Formation Mediated by Silanes in the Presence of Thiols as Polarity- Reversal Catalysts. Chem. Commun. 1998, 1413–1414.
- 49
Cai, Y.; Roberts, B. P.; Tocher, D. A. Carbohydrate-Derived Thiols as Protic Polarity-Reversal Catalysts for Enantioselective Radical-Chain Reactions. J. Chem. Soc., Perkin Trans. 1 2002, 1376–1386.
10.1039/b202022j Google Scholar
- 50 Shi, Q.; Xu, M.; Chang, R.; Ramanathan, D.; Peñín, B.; Funes-Ardoiz, I.; Ye, J. Visible-Light Mediated Catalytic Asymmetric Radical Deuteration at Non-Benzylic Positions. Nat. Commun. 2022, 13, 4453.
- 51 Ramanathan, D.; Shi, Q.; Xu, M.; Chang, R.; Peñín, B.; Funes-Ardoiz, I.; Ye, J. Catalytic Asymmetric Deuterosilylation of Exocyclic Olefins with Mannose-Derived Thiols and Deuterium Oxide. Org. Chem. Front. 2023, 10, 1182–1190.
- 52 Hejna, B. G.; Ganley, J. M.; Shao, H.; Tian, H.; Ellefsen, J. D.; Fastuca, N. J.; Houk, K. N.; Miller, S. J.; Knowles, R. R. Catalytic Asymmetric Hydrogen Atom Transfer: Enantioselective Hydroamination of Alkenes. J. Am. Chem. Soc. 2023, 145, 16118–16129.
- 53 Tang, L.; Shen, C.; Hao, S.; Dong, K. A Type of Chiral C2-Symmetric Arylthiol Catalyst for Highly Enantioselective Anti-Markovnikov Hydroamination. J. Am. Chem. Soc. 2024, 146, 16248−16256.
- 54 Brill, Z. G.; Grover, H. K.; Maimone, T. J. Enantioselective Synthesis of an Ophiobolin Sesterterpene via a Programmed Radical Cascade. Science 2016, 352, 1078–1082.
- 55 Emmanuel, M. A.; Greenberg, N. R.; Oblinsky, D. G.; Hyster, T. K. Accessing Non-Natural Reactivity by Irradiating Nicotinamide-Dependent Enzymes with Light. Nature 2016, 540, 414–417.
- 56 Sandoval, B. A.; Meichan, A. J.; Hyster, T. K. Enantioselective Hydrogen Atom Transfer: Discovery of Catalytic Promiscuity in Flavin-Dependent ‘Ene’-Reductases. J. Am. Chem. Soc. 2017, 139, 11313–11316.
- 57 Biegasiewicz, K. F.; Cooper, S. J.; Emmanuel, M. A.; Miller, D. C.; Hyster, T. K. Catalytic Promiscuity Enabled by Photoredox Catalysis in Nicotinamide-Dependent Oxidoreductases. Nat. Chem. 2018, 10, 770–775.
- 58 Biegasiewicz, K. F.; Cooper, S. J.; Gao, X.; Oblinsky, D. G.; Kim, J. H.; Garfinkle, S. E.; Joyce, L. A.; Sandoval, B. A.; Scholes, G. D.; Hyster, T. K. Photoexcitation of Flavoenzymes Enables a Stereoselective Radical Cyclization. Science 2019, 364, 1166–1169.
- 59 Clayman, P. D.; Hyster, T. K. Photoenzymatic Generation of Unstabilized Alkyl Radicals: An Asymmetric Reductive Cyclization. J. Am. Chem. Soc. 2020, 142, 15673–15677.
- 60 Sandoval, B. A.; Kurtoic, S. I.; Chung, M. M.; Biegasiewicz, K. F.; Hyster, T. K. Photoenzymatic Catalysis Enables Radical-Mediated Ketone Reduction in Ene-Reductases. Angew. Chem. Int. Ed. 2019, 58, 8714–8718.
- 61 Nakano, Y.; Black, M. J.; Meichan, A. J.; Sandoval, B. A.; Chung, M. M.; Biegasiewicz, K. F.; Zhu, T.; Hyster, T. K. Photoenzymatic Hydrogenation of Heteroaromatic Olefins Using 'Ene'-Reductases with Photoredox Catalysts. Angew. Chem. Int. Ed. 2020, 59, 10484–10488.
- 62 Sandoval, B. A.; Clayman, P. D.; Oblinsky, D. G.; Oh, S.; Nakano, Y.; Bird, M.; Scholes, G. D.; Hyster, T. K. Photoenzymatic Reductions Enabled by Direct Excitation of Flavin Dependent “Ene”-Reductases. J. Am. Chem. Soc. 2021, 143, 1735–1739.
- 63 Zhang, J.; Zhang, Q.; Chen, B.; Yu, J.; Wang, B.; Huang, X. Photoenzymatic Conversion of Enamides to Enantioenriched Benzylic Amines Enabled by Visible-Light-Induced Single-Electron Reduction. ACS Catal. 2023, 13, 15682–15690.
- 64 Huang, X.; Wang, B.; Wang, Y.; Jiang, G.; Feng, J.; Zhao, H. Photoenzymatic Enantioselective Intermolecular Radical Hydroalkylation. Nature 2020, 584, 69–74.
- 65 Page, C. G.; Cooper, S. J.; DeHovitz, J. S.; Oblinsky, D. G.; Biegasiewicz, K. F.; Antropow, A. H.; Armbrust, K. W.; Ellis, J. M.; Hamann, L. G.; Horn, E. J.; Oberg, K. M.; Scholes, G. D.; Hyster, T. K. Quaternary Charge-Transfer Complex Enables Photoenzymatic Intermolecular Hydroalkylation of Olefins. J. Am. Chem. Soc. 2021, 143, 97–102.
- 66 Li, M.; Harrison, W.; Zhang, Z.; Yuan, Y.; Zhao, H. Remote Stereocontrol with Azaarenes via Enzymatic Hydrogen Atom Transfer. Nat. Chem. 2024, 16, 277–284.
- 67 Bender, S. G.; Hyster, T. K. Pyridylmethyl Radicals for Enantioselective Alkene Hydroalkylation Using “Ene”-Reductases. ACS Catal. 2023, 13, 14680–14684.
- 68 Huang, X.; Feng, J.; Cui, J.; Jiang, G.; Harrison, W.; Zang, X.; Zhou, J.; Wang, B.; Zhao, H. Photoinduced Chemomimetic Biocatalysis for Enantioselective Intermolecular Radical Conjugate Addition. Nat. Catal. 2022, 5, 586–593.
- 69
Sun, S.-Z.; Nicholls, B. T.; Bain, D.; Qiao, T.; Page, C. G.; Musser, A. J.; Hyster, T. K. Enantioselective Decarboxylative Alkylation Using Synergistic Photoenzymatic Catalysis. Nat. Catal. 2023, 7, 35–42.
10.1038/s41929-023-01065-5 Google Scholar
- 70 Ye, Y.; Cao, J.; Oblinsky, D. G.; Verma, D.; Prier, C. K.; Scholes, G. D.; Hyster, T. K. Using Enzymes to Tame Nitrogen-Centred Radicals for Enantioselective Hydroamination. Nat. Chem. 2023, 15, 206–212.
- 71 Zhang, Z.; Feng, J.; Yang, C.; Cui, H.; Harrison, W.; Zhong, D.; Wang, B.; Zhao, H. Photoenzymatic Enantioselective Intermolecular Radical Hydroamination. Nat. Catal. 2023, 6, 687–694.
- 72 Zhao, B.; Feng, J.; Yu, L.; Xing, Z.; Chen, B.; Liu, A.; Liu, F.; Shi, F.; Zhao, Y.; Tian, C.; Wang, B.; Huang, X. Direct Visible-Light-Excited Flavoproteins for Redox-Neutral Asymmetric Radical Hydroarylation. Nat. Catal. 2023, 6, 996–1004.
- 73 Chen, X.; Zheng, D.; Jiang, L.; Wang, Z.; Duan, X.; Cui, D.; Liu, S.; Zhang, Y.; Yu, X.; Ge, J.; Xu, J. Photoenzymatic Hydrosulfonylation for the Stereoselective Synthesis of Chiral Sulfones. Angew. Chem. Int. Ed. 2023, 62, e202218140.
- 74 Shi, Q.; Kang, X.-W.; Liu, Z.; Sakthivel, P.; Aman, H.; Chang, R.; Yan, X.; Pang, Y.; Dai, S.; Ding, B.; Ye, J. Single-Electron Oxidation-Initiated Enantioselective Hydrosulfonylation of Olefins Enabled by Photoenzymatic Catalysis. J. Am. Chem. Soc. 2024, 146, 2748–2756.
- 75 Fu, H.; Cao, J.; Qiao, T.; Qi, Y.; Charnock, S. J.; Garfinkle, S.; Hyster, T. K. An Asymmetric SP3–SP3 Cross-Electrophile Coupling Using ‘Ene’-Reductases. Nature 2022, 610, 302–307.




