Enantioselective Alkylation of Aldehydes with Organoborons Enabled by Nickel/N-Heterocyclic Carbene Catalysis†
Song-Yang Liu
College of Chemistry and Material Science, Shanghai Normal University, 100 Guilin Road, Shanghai, 200234 China
Search for more papers by this authorCorresponding Author
Zi-Chao Wang
State Key Laboratory of Organometallic Chemistry, Shanghai Institute of Organic Chemistry, University of Chinese Academy of Sciences, Chinese Academy of Sciences, 345 Lingling Road, Shanghai, 200032 China
E-mail: [email protected], [email protected]Search for more papers by this authorCorresponding Author
Shi-Liang Shi
College of Chemistry and Material Science, Shanghai Normal University, 100 Guilin Road, Shanghai, 200234 China
State Key Laboratory of Organometallic Chemistry, Shanghai Institute of Organic Chemistry, University of Chinese Academy of Sciences, Chinese Academy of Sciences, 345 Lingling Road, Shanghai, 200032 China
E-mail: [email protected], [email protected]Search for more papers by this authorSong-Yang Liu
College of Chemistry and Material Science, Shanghai Normal University, 100 Guilin Road, Shanghai, 200234 China
Search for more papers by this authorCorresponding Author
Zi-Chao Wang
State Key Laboratory of Organometallic Chemistry, Shanghai Institute of Organic Chemistry, University of Chinese Academy of Sciences, Chinese Academy of Sciences, 345 Lingling Road, Shanghai, 200032 China
E-mail: [email protected], [email protected]Search for more papers by this authorCorresponding Author
Shi-Liang Shi
College of Chemistry and Material Science, Shanghai Normal University, 100 Guilin Road, Shanghai, 200234 China
State Key Laboratory of Organometallic Chemistry, Shanghai Institute of Organic Chemistry, University of Chinese Academy of Sciences, Chinese Academy of Sciences, 345 Lingling Road, Shanghai, 200032 China
E-mail: [email protected], [email protected]Search for more papers by this authorDedicated to the Special Issue of Nickel Catalysis.
Comprehensive Summary
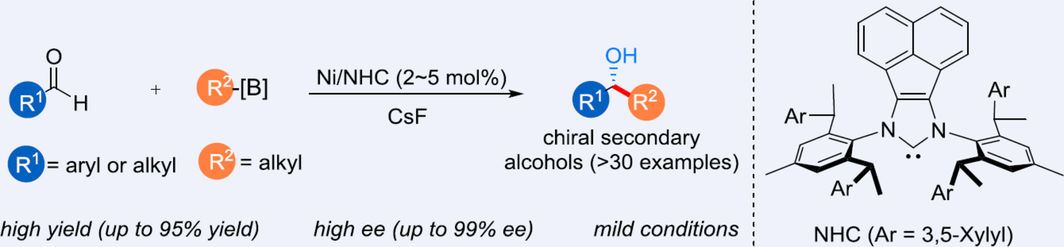
Transition-metal-catalyzed asymmetric alkylation of aldehydes represents a straightforward strategy for the synthesis of chiral secondary alcohols. However, efficient methods using organoborons as coupling reagents are rare. Herein, we report a highly enantioselective nickel-catalyzed alkylation reaction of aldehydes, using readily available alkylborons as nucleophiles. A wide variety of chiral secondary alcohols were prepared from commercially available aldehydes with high yields. The key to the excellent enantioselectivity and chemoselectivity was the employment of a bulky C2-symmetric chiral NHC ligand. This protocol features excellent enantiocontrol, mild conditions, and good functional group compatibility.
Supporting Information
| Filename | Description |
|---|---|
| cjoc202400288-sup-0001-supinfo.pdfPDF document, 7.9 MB |
Appendix S1: Supporting Information |
Please note: The publisher is not responsible for the content or functionality of any supporting information supplied by the authors. Any queries (other than missing content) should be directed to the corresponding author for the article.
References
- 1(a) Mozga, T.; Prokop, Z.; Chaloupková, R.; Damborský, J. Chiral aliphatic hydroxy compounds in nature: A review of biological functions and practical applications. Collect. Czech. Chem. Commun. 2009, 74, 1195–1278; (b) Klaholz, B. P.; Mitschler, A.; Moras, D. Structural Basis for Isotype Selectivity of the Human Retinoic Acid Nuclear Receptor. J. Mol. Biol. 2000, 302, 155–170.
- 2 https://bpb-us-e2.wpmucdn.com/sites.arizona.edu/dist/9/130/files/2023/11/NjardarsonGroup2022SmallMoleculeTopPosterV3.pdf
- 3(a) Zhou, Q.; Srinivas, H. D.; Dasgupta, S.; Watson, M. P. Nickel-Catalyzed Cross-Couplings of Benzylic Pivalates with Arylboroxines: Stereospecific Formation of Diarylalkanes and Triarylmethanes. J. Am. Chem. Soc. 2013, 135, 3307–3310; (b) Harris, M. R.; Hanna, L. E.; Greene, M. A.; Moore, C. E.; Jarvo, E. R. Retention or Inversion in Stereospecific Nickel-Catalyzed Cross-Coupling of Benzylic Carbamates with Arylboronic Esters: Control of Absolute Stereochemistry with an Achiral Catalyst. J. Am. Chem. Soc. 2013, 135, 3303–3306.
- 4
Zhou, Q.-L. Privileged Chiral Ligands and Catalysts, John Wiley & Sons, 2011.
10.1002/9783527635207 Google Scholar
- 5(a) Tang, W.; Zhang, X. New Chiral Phosphorus Ligands for Enantioselective Hydrogenation. Chem. Rev. 2003, 103, 3029–3069; (b) Tian, P.; Dong, H.-Q.; Lin, G.-Q. Rhodium-Catalyzed Asymmetric Arylation. ACS Catal. 2012, 2, 95–119; (c) Noyori, R.; Ohkuma, T. Asymmetric Catalysis by Architectural and Functional Molecular Engineering: Practical Chemo- and Stereoselective Hydrogenation of Ketones. Angew. Chem. Int. Ed. 2001, 40, 40–73; (d) Jiang, B.; Shi, S.-L. Recent Progress in Upgrading of Alcohol and Amine via Asymmetric Dehydrogenative Coupling. Chin. J. Org. Chem. 2022, 42, 3263–3279; (e) Wu, Z.-J.; Wu, Z.; Zhang, W.-W.; Gu, Q.; You, S.-L. Rh(III)-Catalyzed Enantioselective Intermolecular Aryl C—H Bond Addition to Aldehydes. Chin. J. Chem. 2022, 40, 2780–2784; (f) Tang, X.; Su, Z.; Lin, Q.; Lin, L.; Dong, S.; Feng, X. Asymmetric Catalytic α-Selective Allylation of Ketones with Allyltrifluoroborates Using Dual-Functional Chiral InIII/N,N′-Dioxide Complex. Chin. J. Chem. 2022, 40, 1793–1798; (g) Qian, L.; Tang, X.; Wang, Y.; Liu, G.; Huang, Z. Asymmetric Transfer Hydrogenation of Diaryl Ketones with Ethanol Catalyzed by Chiral NCP Pincer Iridium Complexes. Chin. J. Chem. 2022, 40, 1131–1136.
- 6For review on asymmetric nucleophilic addition of organometallic reagents to aldehyde: (a) Luo, W.; Zhang, L. M.; Zhang, Z. M.; Zhang, J. Synthesis of W-Phos Ligand and Its Application in the Copper-Catalyzed Enantioselective Addition of Linear Grignard Reagents to Ketones. Angew. Chem. Int. Ed. 2022, 61, e202204443; (b) Harada, T. Development of highly active chiral titanium catalysts for the enantioselective addition of various organometallic reagents to aldehydes. Chem. Rec. 2016, 16, 1256–1273; (c) Teixeira, W. K.; de Albuquerque, D. Y.; Narayanaperumal, S.; Schwab, R. S. Recent advances in the synthesis of enantiomerically enriched diaryl, aryl heteroaryl, and diheteroaryl alcohols through addition of organometallic reagents to carbonyl compounds. Synthesis 2020, 52, 1855–1873; (d) Hatano, M.; Miyamoto, T.; Ishihara, K. Recent Progress in Selective Additions of Organometal Reagents to Carbonyl Compounds. Curr. Org. Chem. 2007, 11, 127–157; (e) Noyori, R.; Kitamura, M. Enantioselective Addition of Organometallic Reagents to Carbonyl Compounds: Chirality Transfer, Multiplication, and Amplification. Angew. Chem. Int. Ed. 1991, 30, 49–69.
- 7Selected examples on asymmetric nucleophilic addition of organomagnesium reagents to aldehydes: (a) Collados, J. F.; Solà, R.; Harutyunyan, S. R.; Maciá, B. Catalytic synthesis of enantiopure chiral alcohols via addition of Grignard reagents to carbonyl compounds. ACS Catal. 2016, 6, 1952–1970;
(b) Muramatsu, Y.; Harada, T. Catalytic asymmetric alkylation of aldehydes with Grignard reagents. Angew. Chem. Int. Ed. 2008, 120, 1104–1106;
10.1002/ange.200704963 Google Scholar(c) Liu, Y.; Da, C. S.; Yu, S. L.; Yin, X. G.; Wang, J. R.; Fan, X. Y.; Li, W.-P.; Wang, R. Catalytic highly enantioselective alkylation of aldehydes with deactivated Grignard reagents and synthesis of bioactive intermediate secondary arylpropanols. J. Org. Chem. 2010, 75, 6869–6878; (d) Muramatsu, Y.; Kanehira, S.; Tanigawa, M.; Miyawaki, Y.; Harada, T. Catalytic enantioselective alkylation and arylation of aldehydes by using Grignard reagents. Bull. Chem. Soc. Jpn. 2010, 83, 19–32; (e) Fernández- Mateos, E.; Maciá, B.; Yus, M. Catalytic Enantioselective Addition of Alkyl Grignard Reagents to Aliphatic Aldehydes. Adv. Synth. Catal. 2013, 355, 1249–1254; (f) Tanaka, K.; Tomihama, M.; Yamamoto, K.; Matsubara, N.; Harada, T. Method for Catalytic Enantioselective Alkylation of Aldehydes Using Grignard Reagents as Alkyl Sources. J. Org. Chem. 2018, 83, 6127–6132; (g) Hatano, M.; Gouzu, R.; Mizuno, T.; Abe, H.; Yamada, T.; Ishihara, K. Catalytic enantioselective alkyl and aryl addition to aldehydes and ketones with organozinc reagents derived from alkyl Grignard reagents or arylboronic acids. Catal. Sci. Technol. 2011, 1, 1149–1158.
- 8Selected examples on asymmetric nucleophilic addition of organo- zinc reagents to aldehydes: (a) Binder, C. M.; Singaram, B. Asymmetric addition of diorganozinc reagents to aldehydes and ketones. Org. Prep. Proced. Int. 2011, 43, 139–208; (b) Kinoshita, Y.; Kanehira, S.; Hayashi, Y.; Harada, T. Catalytic Enantioselective Alkylation of Aldehydes by Using Organozinc Halide Reagents. Chem. Eur. J. 2013, 19, 3311–3314; (c) Pu, L. Asymmetric Functional Organozinc Additions to Aldehydes Catalyzed by 1,10-Bi-2-naphthols (BINOLs). Acc. Chem. Res. 2014, 47, 1523–1535; (d) Cozzi, P. G.; Kotrusz, P. Highly enantioselective addition of Me2Zn to aldehydes catalyzed by ClCr (Salen) J. Am. Chem. Soc. 2006, 128, 4940–4941; (e) Nugent, W. A. An amino alcohol ligand for highly enantioselective addition of organozinc reagents to aldehydes: Serendipity rules. Org. Lett. 2002, 4, 2133–2136.
- 9Selected examples on asymmetric nucleophilic addition of organolithium reagents to aldehydes: (a) Luderer, M. R.; Bailey, W. F.; Luderer, M. R.; Fair, J. D.; Dancer, R. J.; Sommer, M. B. Asymmetric addition of achiral organomagnesium reagents or organolithiums to achiral aldehydes or ketones: A review. Tetrahedron Asymmetry 2009, 20, 981–998; (b) Rönnholm, P., Södergren, M.; Hilmersson, G. Improved and efficient synthesis of chiral N, P-Ligands via cyclic sulfamidates for asymmetric addition of butyllithium to benzaldehyde. Org. Lett. 2007, 9, 3781–3783; (c) Veguillas M.; Sola R.; Shaw, L.; Macia, B. Catalytic asymmetric addition of organolithium reagents to aldehydes. Eur. J. Org. Chem. 2016, 2016, 1788–1794.
- 10
Hall, D. G. Boronic Acids: Preparation and Applications in Organic Synthesis and Medicine, Wiley-VCH, Weinheim, Germany, 2005.
10.1002/3527606548 Google Scholar
- 11For enantioselective arylation of aldehydes with organoborons, see: (a) Wang, Z.-C.; Gao, J.; Cai, Y.; Ye, X.; Shi, S.-L. Chemo-and Enantioselective Arylation and Alkenylation of Aldehydes Enabled by Nickel/N-Heterocyclic Carbene Catalysis. CCS Chem. 2022, 4, 1169–1179; (b) Zhang, S.; Perveen, S.; Ouyang, Y.; Xu, L.; Yu, T.; Zhao, M., Wang, L.; Song, P.; Li, P. Design and Synthesis of Tunable Chiral 2,2’-Bipyridine Ligands: Application to the Enantioselective Nickel- Catalyzed Reductive Arylation of Aldehydes. Angew. Chem. Int. Ed. 2022, 61, e202117843; (c) Jiang, H.; He, X. K.; Jiang, X.; Zhao, W.; Lu, L. Q.; Cheng, Y.; Xiao, W. J. Photoinduced Cobalt-Catalyzed Desymmetrization of Dialdehydes to Access Axial Chirality. J. Am. Chem. Soc. 2023, 145, 6944–6952.
- 12For enantioselective allylation of aldehydes with organoborons, see: (a) Hirayama, L. C.; Haddad, T. D.; Oliver, A. G.; Singaram, B. Direct synthesis of B-allyl and B-allenyldiisopinocampheylborane reagents using allyl or propargyl halides and indium metal under Barbier-type conditions. J. Org. Chem. 2012, 77, 4342–43e53; (b) Wu, T. R.; Shen, L.; Chong, J. M. Asymmetric allylboration of aldehydes and ketones using 3, 3 ‘-disubstitutedbinaphthol-modified boronates. Org. Lett. 2004, 6, 2701–2704.
- 13(a) Netherton, M. R.; Dai, C.; Neuschuetz, K.; Fu, G. C. Room-Temperature Alkyl-Alkyl Suzuki Cross-Coupling of Alkyl Bromides that Possess β Hydrogens. J. Am. Chem. Soc. 2001, 123, 10099–10100; (b) Kirchhoff, J. H.; Netherton; M. R.; Hills, I. D.; Fu, G. C. Boronic Acids: New Coupling Partners in Room-Temperature Suzuki Reactions of Alkyl Bromides. Crystallographic Characterization of an Oxidative- Addition Adduct Generated under Remarkably Mild Conditions. J. Am. Chem. Soc. 2002, 124, 13662–13663.
- 14(a) Ukon, T.; Harada, T. Catalytic asymmetric alkylation of aldehydes by using trialkylboranes. Eur. J. Org. Chem. 2008, 4405–4407; (b) Akai, J.; Watanabe, S.; Michikawa, K.; Harada, T. Application of a Heterogeneous Chiral Titanium Catalyst Derived from Silica-Supported 3-Aryl H8-BINOL to Enantioselective Alkylation and Arylation of Aldehydes. Org. Lett. 2017, 19, 3632–3635.
- 15 Kumar, R.; Kawasaki, H.; Harada, T. Enantioselective Alkylation of Aldehydes Using Functionalized Alkylboron Reagents Catalyzed by a Chiral Titanium Complex. Org. Lett. 2013, 15, 4198–4201.
- 16(a) Cai, Y.; Yang, X.-T.; Zhang, S.-Q.; Li, F.; Li, Y.-Q.; Ruan, L.-X.; Hong, X. Shi, S.-L. Copper-Catalyzed Enantioselective Markovnikov Protoboration of α-Olefins Enabled by a Buttressed N-Heterocyclic Carbene Ligand. Angew. Chem. Int. Ed. 2018, 57, 1376–1380; (b) Shen, D.; Xu, Y.; Shi, S.-L. A Bulky Chiral N-Heterocyclic Carbene Palladium Catalyst Enables Highly Enantioselective Suzuki-Miyaura Cross-Coupling Reactions for the Synthesis of Biaryl Atropisomers. J. Am. Chem. Soc. 2019, 141, 14938–14945; (c) Wang, Z.-C.; Xie, P.-P.; Xu, Y.; Hong, X.; Shi, S.-L. Low-Temperature Nickel-Catalyzed C−N Cross-Coupling via Kinetic Resolution Enabled by a Bulky and Flexible Chiral N-Heterocyclic Carbene Ligand. Angew. Chem. Int. Ed. 2021, 60, 16077–16220; (d) Cai, Y.; Shi, S.-L. Enantioconvergent Arylation of Racemic Secondary Alcohols to Chiral Tertiary Alcohols Enabled by Nickel/N-Heterocyclic Carbene Catalysis J. Am. Chem. Soc. 2021, 143, 11963–11968; (e) Zhang, W.-B.; Chen, G.; Shi, S.-L. Enantioselective Ni/N-Heterocyclic Carbene-Catalyzed Redox-Economical Coupling of Aldehydes, Alkynes, and Enones for Rapid Construction of Acyclic All-Carbon Quaternary Stereocenters. J. Am. Chem. Soc. 2022, 144, 130–136; (f) Cai, Y.; Ruan, L.-X.; Rahman, A.; Shi, S.-L. Fast Enantio- and Chemoselective Arylation of Ketones with Organoboronic Esters Enabled by Nickel/NHC Catalysis. Angew. Chem. Int. Ed. 2021, 60, 5262–5267; (g) Ruan, L.-X.; Sun, B.; Liu, J.-M.; Shi, S.-L. Dynamic kinetic asymmetric arylation and alkenylation of ketones. Science 2023, 379, 662–670.
- 17(a) Hoshimoto, Y.; Ohashi, M.; Ogoshi, S. Catalytic transformation of aldehydes with nickel complexes through η2 coordination and oxidative cyclization. Acc. Chem. Res. 2015, 48, 1746–1755; (b) Bouffard, J.; Itami, K. A nickel catalyst for the addition of organoboronate esters to ketones and aldehydes. Org. Lett. 2009, 11, 4410–4413.




