Stereoselective Synthesis of 2-Deoxy-α-N-Glycosides from Glycals with 1,4,2-Dioxazol-5-ones
Zhenpeng Shen
The Institute for Advanced Studies, Wuhan University, Wuhan, Hubei, 430072 China
Search for more papers by this authorCorresponding Author
Guoyin Yin
The Institute for Advanced Studies, Wuhan University, Wuhan, Hubei, 430072 China
E-mail: [email protected]; [email protected]Search for more papers by this authorCorresponding Author
Yangyang Li
The Institute for Advanced Studies, Wuhan University, Wuhan, Hubei, 430072 China
E-mail: [email protected]; [email protected]Search for more papers by this authorZhenpeng Shen
The Institute for Advanced Studies, Wuhan University, Wuhan, Hubei, 430072 China
Search for more papers by this authorCorresponding Author
Guoyin Yin
The Institute for Advanced Studies, Wuhan University, Wuhan, Hubei, 430072 China
E-mail: [email protected]; [email protected]Search for more papers by this authorCorresponding Author
Yangyang Li
The Institute for Advanced Studies, Wuhan University, Wuhan, Hubei, 430072 China
E-mail: [email protected]; [email protected]Search for more papers by this authorComprehensive Summary
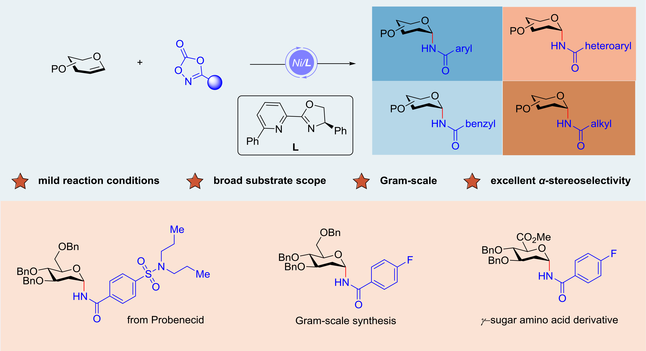
The synthesis of N-glycosides has received significant attention due to their crucial role in carbohydrate chemistry. Despite considerable advancements developed in the construction of N-glycosides, methods for the stereoselective construction of 2-deoxy-α-N-glycosides are still limited. Herein, we disclosed a nickel-catalyzed hydroamination of glycals under mild conditions. This transformation could allow for the stereoselective synthesis of an array of 2-deoxy-α-N-glycosides with excellent α-stereoselectivity. Nickel-catalyzed glycosylation reactions, particularly those involving anomeric C(sp3)-metal bond formation, have proven to be an effective and stereoselective strategy for producing various N-glycosides. Additionally, with highlight of the application of this reaction, γ-sugar amino acid derivatives were synthesized.
Supporting Information
| Filename | Description |
|---|---|
| cjoc_202400224_sm_suppl.pdfPDF document, 6.2 MB |
Appendix S1: Supporting Information |
Please note: The publisher is not responsible for the content or functionality of any supporting information supplied by the authors. Any queries (other than missing content) should be directed to the corresponding author for the article.
References
- 1(a) Varki, A. Biological Roles of Oligosaccharides: All of the Theories Are Correct. Glycobiology 1993, 3, 97—130;
(b) Davis, B. G. Recent developments in glycoconjugates. J. Chem. Soc., Perkin Trans. 1 1999, 3215–3237;
10.1039/a809773i Google Scholar(c) Rudd, P. M.; Elliott, T.; Cresswell, P.; Wilson, I. A.; Dwek, R. A. Glycosylation and the Immune System. Science 2001, 291, 2370–2376; (d) Bertozzi, C. R.; Kiessling, L. L. Chemical Glycobiology. Science 2001, 291, 2357–2364; (e) Seeberger, P. H.; Werz, D. B. Synthesis and medical applications of oligosaccharides. Nature 2007, 446, 1046–1051; (f) Zhu, D.; Yu, B. Synthesis of the Diverse Glycosides in Traditional Chinese Medicine. Chin. J. Chem. 2018, 36, 681–691.
- 2(a) Feizi, T. Demonstration by monoclonal antibodies that carbohydrate structures of glycoproteins and glycolipids are onco-developmental antigens. Nature 1985, 314, 53–57; (b) Varki, A. Glycan-based interactions involving vertebrate sialic-acid-recognizing proteins. Nature 2007, 446, 1023–1029; (c) Adams, M. M.; Damani, P.; Perl, N. R.; Won, A.; Hong, F.; Livingston, P. O.; Ragupathi, G.; Gin, D. Y. Design and Synthesis of Potent Quillaja Saponin Vaccine Adjuvants. J. Am. Chem. Soc. 2010, 132, 1939–1945.
- 3(a) Davis, B. G. Synthesis of Glycoproteins. Chem. Rev. 2002, 102, 579–602;
(b) St. Hilaire, P. M.; Meldal, M. Glycopeptide and Oligosaccharide Libraries. Angew. Chem. Int. Ed. 2000, 39, 1162–1179;
10.1002/(SICI)1521-3773(20000403)39:7<1162::AID-ANIE1162>3.0.CO;2-6 PubMed Web of Science® Google Scholar(c) Boons, G. J.; Demchenko, A. V. Recent Advances in O-Sialylation. Chem. Rev. 2000, 100, 4539–4566; (d) Paleček, E.; Tkáč, J.; Bartošík, M.; Bertók, T.; Ostatná, V.; Paleček, J. Electrochemistry of Nonconjugated Proteins and Glycoproteins. Toward Sensors for Biomedicine and Glycomics. Chem. Rev. 2015, 115, 2045–2108; (e) Corcilius, L.; Payne, R. J. Stereoselective Synthesis of Sialylated Tumor-Associated Glycosylamino Acids. Org. Lett. 2013, 15, 5794–5797; (f) Dube, D. H.; Bertozzi, C. R. Glycans in cancer and inflammation – potential for therapeutics and diagnostics. Nat. Rev. Drug Discov. 2005, 4, 477–488; (g) Gaidzik, N.; Westerlind, U.; Kunz, H. The development of synthetic antitumour vaccines from mucin glycopeptide antigens. Chem. Soc. Rev. 2013, 42, 4421–4442; (h) Wilson, R. M.; Danishefsky, S. J. A Vision for Vaccines Built from Fully Synthetic Tumor-Associated Antigens: From the Laboratory to the Clinic. J. Am. Chem. Soc. 2013, 135, 14462–14472; (i) Brocke, C.; Kunz, H. Synthesis of Tumor- Associated Glycopeptide Antigens. Bioorg. Med. Chem. 2002, 10, 3085–3112; (j) Payne, R. J.; Wong, C.-H. Advances in chemical ligation strategies for the synthesis of glycopeptides and glycoproteins. Chem. Commun. 2010, 46, 21–43.
- 4(a) Shibata, S.; Miyakawa, Y.; Naruse, T.; Nagasawa, T.; Takuma, T. A glycoprotein that induces nephrotoxic antibody: its isolation and purification from rat glomerular basement membrane. J. Immunol. 1969, 102, 593–601; (b) Shibata, S.; Nagasawa, T.; Miyakawa, Y.; Naruse, T. Nephritogenic glycoprotein: I. Proliferative glomerulonephritis induced in rats by a single injection of the soluble glycoprotein isolated from homologous glomerular basement membrane. J. Immunol. 1971, 106, 1284–1294; (c) Sasaki, M.; Tachibana, K.; Nakanishi, H. An efficient and stereocontrolled synthesis of the nephritogenoside core structure. Tetrahedron Lett. 1991, 32, 6873–6876; (d) Takeda, T.; Utsuno, A.; Okamoto, N.; Ogihara, Y.; Shibata, S. Synthesis of the α and β anomer of an N-triglycosyl dipeptide. Carbohydr. Res. 1990, 207, 71–79; (e) McDonagh, A. W.; Murphy, P. V. Synthesis of α-galactosyl ceramide analogues with an α-triazole at the anomeric carbon. Tetrahedron 2014, 70, 3191–3196; (f) Helenius, A.; Aebi, M. Roles of N-Linked Glycans in the Endoplasmic Reticulum. Annu. Rev. Biochem. 2004, 73, 1019–1049.
- 5(a) Kobayashi, Y.; Miyazaki, H.; Shiozaki, M. Syntheses of Trehazolin, Trehalamine, and the Aminocyclitol Moiety of Trehazolin: Determination of Absolute Configuration of Trehazolin. J. Org. Chem. 1994, 59, 813–822; (b) Ledford, B. E.; Carreira, E. M. Total Synthesis of (+)-Trehazolin: Optically Active Spirocycloheptadienes as Useful Precursors for the Synthesis of Amino Cyclopentitols. J. Am. Chem. Soc. 1995, 117, 11811–11812; (c) Li, J.; Lang, F.; Ganem, B. Enantioselective Approaches to Aminocyclopentitols: A Total Synthesis of (+)-6-Epitrehazolin and a Formal Total Synthesis of (+)-Trehazolin. J. Org. Chem. 1998, 63, 3403–3410; (d) Boiron, A.; Zillig, P.; Faber, D.; Giese, B. Synthesis of Trehazolin from D-Glucose. J. Org. Chem. 1998, 63, 5877–5882; (e) Berecibar, A.; Grandjean, C.; Siriwardena, A. Synthesis and biological activity of natural aminocyclopentitol glycosidase inhibitors: mannostatins, trehazolin, allosamidins, and their analogues. Chem. Rev. 1999, 99, 779–844; (f) Kobayashi, Y. Chemistry and biology of trehazolins. Carbohydr. Res. 1999, 315, 3–15.
- 6(a) Li, J. S.; Cui, L.; Rock, D. L.; Li, J. Novel Glycosidic Linkage in Aedes aegypti Chorion Peroxidase: N-MANNOSYL TRYPTOPHAN. J. Biol. Chem. 2005, 280, 38513–38521;
(b) Lin, C.-K.; Yun, W.-Y.; Lin, L.-T.; Cheng, W.-C. A concise approach to the synthesis of the unique N-mannosyl D-β-hydroxyenduracididine moiety in the mannopeptimycin series of natural products. Org. Biomol. Chem. 2016, 14, 4054–4060;
(c) Manabe, S.; Ito, Y. The first synthesis of N-Man-Trp: Alternative mannosylation modification of protein. Synlett 2008, 2008, 880–882.
10.1055/s-2008-1032099 Google Scholar
- 7(a) Arsequell, G.; Valencia, G. Recent advances in the synthesis of complex N-glycopeptides. Tetrahedron: Asymmetry 1999, 10, 3045–3094; (b) Ratcliffe, A. J.; Konradsson, P.; Fraser-Reid, B. N-Pentenyl glycosides as efficient synthons for promoter-mediated assembly of N-α-linked glycoproteins. J. Am. Chem. Soc. 1990, 112, 5665–5667; (c) Damkaci, F.; DeShong, P. Stereoselective Synthesis of α- and β-Glycosylamide Derivatives from Glycopyranosyl Azides via Isoxazoline Intermediates. J. Am. Chem. Soc. 2003, 125, 4408–4409.
- 8(a) Noronkoski, T.; Stoineva, I. B.; Ivanov, I. P.; Petkov, D. D.; Mononen, I. Glycosylasparaginase-catalyzed Synthesis and Hydrolysis of β-Aspartyl Peptides. J. Biol. Chem. 1998, 273, 26295–26297; (b) Kuhn, P.; Guan, C.; Cui, T.; Tarentino, A. L.; Plummer, T. H.; Van Roey, P. Active Site and Oligosaccharide Recognition Residues of Peptide-N4-(N- acetyl-β-D-glucosaminyl)asparagine Amidase F. J. Biol. Chem. 1995, 270, 29493–29497; (c) Fan, J.-Q.; Lee, Y. C. Detailed Studies on Substrate Structure Requirements of Glycoamidases A and F. J. Biol. Chem. 1997, 272, 27058–27064; (d) Laupichle, L.; Sowa, C. E.; Thiem, J. Synthesis and structural studies of asparagine-modified 2-deoxy-α-N-glycopeptides associated with the renin-Angiotensin system. Bioorg. Med. Chem. 1994, 2, 1281–1294; (e) Bennett, C. S.; Galan, M. C. Methods for 2-Deoxyglycoside Synthesis. Chem. Rev. 2018, 118, 7931–7985; (f) Dai, Y.; Zheng, J.; Zhang, Q. General Strategy for Stereoselective Synthesis of β-N-Glycosyl Sulfonamides via Palladium-Catalyzed Glycosylation. Org. Lett. 2018, 20, 3923–3927; (g) Yang, J.; Dai, Y.; Bartlett, R.; Zhang, Q.; Convergent Palladium- Catalyzed Stereospecific Arginine Glycosylation Using Glycals. Org. Lett. 2021, 23, 4008–4012.
- 9(a) Rawal, G. K.; Kumar, A.; Tawar, U.; Vankar, Y. D. New Method for Chloroamidation of Olefins. Application in the Synthesis of N-Glycopeptides and Anticancer Agents. Org. Lett. 2007, 9, 5171–5174; (b) Meyerhoefer, T. J.; Kershaw, S.; Caliendo, N.; Eltayeb, S.; Hanawa-Romero, E.; Bykovskaya, P.; Huang, V.; Marzabadi, C. H.; De Castro, M. A Practical Synthesis of Various 2-Deoxy-N-glycosides by Using D-Glucal. Eur. J. Org. Chem. 2015, 2015, 2457–2462.
- 10(a) Sherry, B. D.; Loy, R. N.; Toste, F. D. Rhenium(V)-Catalyzed Synthesis of 2-Deoxy-α-glycosides. J. Am. Chem. Soc. 2004, 126, 4510–4511; (b) Colinas, P. A.; Bravo, R. D. A Novel Sulfonamidoglycosylation of Glycals. Org. Lett. 2003, 5, 4509–4511; (c) Bradshaw, G. A.; Colgan, A. C.; Allen, N. P.; Pongener, I.; Boland, M. B.; Ortin, Y.; McGarrigle, E. M. Stereoselective organocatalyzed glycosylations – thiouracil, thioureas and monothiophthalimide act as Brønsted acid catalysts at low loadings. Chem. Sci. 2019, 10, 508–514; (d) Nakatsuji, Y.; Kobayashi, Y.; Takemoto, Y. Direct Addition of Amides to Glycals Enabled by Solvation-Insusceptible 2-Haloazolium Salt Catalysis. Angew. Chem. Int. Ed. 2019, 58, 14115―14119.
- 11(a) Owens, J. M.; Yeung, B. K. S.; Hill, D. C.; Petillo, P. A. Facile C1 Epimerization of α-1-Sulfonamidyl-2-deoxy-2-iodo-glycopyranosides. J. Org. Chem. 2001, 66, 1484–1486;
(b) Chennamadhavuni, D.; Howell, A. R. A solvent-free approach to glycosyl amides: toward the synthesis of α-N-galactosyl ceramides. Tetrahedron Lett. 2015, 56, 3583–3586;
(c) Li, S.; Kobayashi, Y.; Takemoto, Y. Organocatalytic Direct α-Selective N-Glycosylation of Amide with Glycosyl Trichloroacetimidate. Chem. Pharm. Bull. 2018, 66, 768–770;
(d) Kobayashi, Y.; Nakatsuji, Y.; Li, S.; Tsuzuki, S.; Takemoto, Y. Direct N-Glycofunctionalization of Amides with Glycosyl Trichloroacetimidate by Thiourea/Halogen Bond Donor Co-Catalysis. Angew. Chem. Int. Ed. 2018, 57, 3646–3650;
(e) Batchelor, R. J.; Green, D. F.; Johnston, B. D.; Patrick, B. O.; Pinto, B. M. Conformational preferences in glycosylamines. Implications for the exo-anomeric effect. Carbohydr. Res. 2001, 330, 421–426;
(f) Lavecchia, M. J.; Rodríguez, O. M.; Echeverría, G. A.; Pis Diez, R.; Colinas, P. A. Conformational behavior of peracetylated β-d-mannopyranosyl methanesulfonamide: implications for the mechanism of sulfonamidoglycosylation of carbohydrate derivatives. Carbohydr. Res. 2012, 361, 182–188;
(g) An, S.; Wang, Q.; Zhu, W.; Sun, Q.; He, G.; Chen, G. Palladium-Catalyzed O- and N-Glycosylation with Glycosyl Chlorides. CCS Chem. 2020, 3, 1821–1829.
10.31635/ccschem.020.202000445 Google Scholar
- 12(a) Zhu, F.; Walczak, M. A. Stereochemistry of Transition Metal Complexes Controlled by the Metallo-Anomeric Effect. J. Am. Chem. Soc. 2020, 142, 15127–15136;
(b) Jiang, Y.; Zhang, Y.; Lee, B. C.; Koh, M. J. Diversification of Glycosyl Compounds via Glycosyl Radicals. Angew. Chem. Int. Ed. 2023, 62, e202305138;
(c) Chen, A.; Yang, B.; Zhou, Z.; Zhu, F. Recent advances in transition-metal-catalyzed glycosyl cross- coupling reactions. Chem. Catal. 2022, 2, 3430–3470;
10.1016/j.checat.2022.10.019 Google Scholar(d) Lu, K.; Ma, Y.; Liu, S.; Guo, S.; Zhang, Y. Highly Stereoselective C-Glycosylation by Photocatalytic Decarboxylative Alkynylation on Anomeric Position: A Facile Access to Alkynyl C-Glycosides. Chin. J. Chem. 2022, 40, 681–686; (e) Chen, A.; Xu, L.; Zhou, Z.; Zhao, S.; Yang, T.; Zhu, F. Recent advances in glycosylation involving novel anomeric radical precursors. J. Carbohydr. Chem. 2021, 40, 361–400; (f) Zhu, W.; Sun, Q.; Chang, H.; Zhang, H.-X.; Wang, Q.; Chen, G.; He, G. Synthesis of 2-Deoxy-C-Glycosides via Iridium-Catalyzed sp2 and sp3 C-H Glycosylation with Unfunctionalized Glycals. Chin. J. Chem. 2022, 40, 571–576; (g) Shang, W.; Shi, R.; Niu, D. C-Glycoside Synthesis Enabled by Nickel Catalysis. Chin. J. Chem. 2023, 41, 2217–2236.
- 13(a) Lyu, X.; Zhang, J.; Kim, D.; Seo, S.; Chang, S. Merging NiH Catalysis and Inner-Sphere Metal-Nitrenoid Transfer for Hydroamidation of Alkynes. J. Am. Chem. Soc. 2021, 143, 5867–5877; (b) Choi, H.; Lyu, X.; Kim, D.; Seo, S.; Chang, S. Endo-Selective Intramolecular Alkyne Hydroamidation Enabled by NiH Catalysis Incorporating Alkenylnickel Isomerization. J. Am. Chem. Soc. 2022, 144, 10064–10074; (c) Du, B.; Ouyang, Y.; Chen, Q.; Yu, W.-Y. Thioether-Directed NiH-Catalyzed Remote γ-C(sp3)–H Hydroamidation of Alkenes by 1,4,2-Dioxazol-5- ones. J. Am. Chem. Soc. 2021, 143, 14962–14968; (d) Du, B.; Chan, C.-M.; Ouyang, Y.; Chan, K.; Lin, Z.; Yu, W.-Y. NiH-catalyzed anti-Markovnikov hydroamidation of unactivated alkenes with 1,4,2-dioxazol-5-ones for the direct synthesis of N-alkyl amides. Commun. Chem. 2022, 5, 176; (e) Meng, L.; Yang, J.; Duan, M.; Wang, Y.; Zhu, S. Facile Synthesis of Chiral Arylamines, Alkylamines and Amides by Enantioselective NiH-Catalyzed Hydroamination. Angew. Chem. Int. Ed. 2021, 60, 23584–23589; (f) Zhang, Y.; Qiao, D.; Duan, M.; Wang, Y.; Zhu, S. Enantioselective synthesis of α-aminoboronates by NiH-catalysed asymmetric hydroamidation of alkenyl boronates. Nat. Commun. 2022, 13, 5630.
- 14(a) McDevitt, J. P.; Lansbury, P. T. Glycosamino Acids: New Building Blocks for Combinatorial Synthesis. J. Am. Chem. Soc. 1996, 118, 3818–3828; (b) Schweizer, F. Glycosamino Acids: Building Blocks for Combinatorial Synthesis–Implications for Drug Discovery. Angew. Chem. Int. Ed. 2002, 41, 230–253; (c) Gruner, S. A. W.; Locardi, E.; Lohof, E.; Kessler, H. Carbohydrate-Based Mimetics in Drug Design: Sugar Amino Acids and Carbohydrate Scaffolds. Chem. Rev. 2002, 102, 491–514; (d) Tian, G.-Z.; Wang, X.-L.; Hu, J.; Wang, X.-B.; Guo, X.-Q.; Yin, J. Recent progress of sugar amino acids: Synthetic strategies and applications as glycomimetics and peptidomimetics. Chin. Chem. Lett. 2015, 26, 922–930.




