Asymmetric Synthesis of Dihydrospirotryprostatin B via a Silica Gel-Mediated Cyclization of Tryptamine-Ynamide
Changhong Han
Key Laboratory of Structure-Based Drug Design and Discovery of Ministry of Education, Shenyang Pharmaceutical University, Shenyang, Liaoning, 110016 China
Wuya College of Innovation, Shenyang Pharmaceutical University, Shenyang, Liaoning, 110016 China
Institute of Drug Research in Medicine Capital of China, Benxi, Liaoning, 117000 China
These authors contributed equally to this work.
Search for more papers by this authorLu Yang
Key Laboratory of Structure-Based Drug Design and Discovery of Ministry of Education, Shenyang Pharmaceutical University, Shenyang, Liaoning, 110016 China
Wuya College of Innovation, Shenyang Pharmaceutical University, Shenyang, Liaoning, 110016 China
Institute of Drug Research in Medicine Capital of China, Benxi, Liaoning, 117000 China
These authors contributed equally to this work.
Search for more papers by this authorKaizong Yao
Key Laboratory of Structure-Based Drug Design and Discovery of Ministry of Education, Shenyang Pharmaceutical University, Shenyang, Liaoning, 110016 China
Wuya College of Innovation, Shenyang Pharmaceutical University, Shenyang, Liaoning, 110016 China
Institute of Drug Research in Medicine Capital of China, Benxi, Liaoning, 117000 China
Search for more papers by this authorSen Zhang
Key Laboratory of Structure-Based Drug Design and Discovery of Ministry of Education, Shenyang Pharmaceutical University, Shenyang, Liaoning, 110016 China
Wuya College of Innovation, Shenyang Pharmaceutical University, Shenyang, Liaoning, 110016 China
Institute of Drug Research in Medicine Capital of China, Benxi, Liaoning, 117000 China
Search for more papers by this authorJiayue Fu
Key Laboratory of Structure-Based Drug Design and Discovery of Ministry of Education, Shenyang Pharmaceutical University, Shenyang, Liaoning, 110016 China
Wuya College of Innovation, Shenyang Pharmaceutical University, Shenyang, Liaoning, 110016 China
Institute of Drug Research in Medicine Capital of China, Benxi, Liaoning, 117000 China
Search for more papers by this authorHanyang Sun
Key Laboratory of Structure-Based Drug Design and Discovery of Ministry of Education, Shenyang Pharmaceutical University, Shenyang, Liaoning, 110016 China
Wuya College of Innovation, Shenyang Pharmaceutical University, Shenyang, Liaoning, 110016 China
Institute of Drug Research in Medicine Capital of China, Benxi, Liaoning, 117000 China
Search for more papers by this authorHongsheng Xu
Zhejiang Shapuaisi Pharmaceutical Co., Ltd., Pinghu, Zhejiang, 314299 China
Search for more papers by this authorBin Lin
Key Laboratory of Structure-Based Drug Design and Discovery of Ministry of Education, Shenyang Pharmaceutical University, Shenyang, Liaoning, 110016 China
Wuya College of Innovation, Shenyang Pharmaceutical University, Shenyang, Liaoning, 110016 China
Institute of Drug Research in Medicine Capital of China, Benxi, Liaoning, 117000 China
Search for more papers by this authorMaosheng Cheng
Key Laboratory of Structure-Based Drug Design and Discovery of Ministry of Education, Shenyang Pharmaceutical University, Shenyang, Liaoning, 110016 China
Institute of Drug Research in Medicine Capital of China, Benxi, Liaoning, 117000 China
Search for more papers by this authorCorresponding Author
Yongxiang Liu
Key Laboratory of Structure-Based Drug Design and Discovery of Ministry of Education, Shenyang Pharmaceutical University, Shenyang, Liaoning, 110016 China
Wuya College of Innovation, Shenyang Pharmaceutical University, Shenyang, Liaoning, 110016 China
Institute of Drug Research in Medicine Capital of China, Benxi, Liaoning, 117000 China
E-mail: [email protected]Search for more papers by this authorChanghong Han
Key Laboratory of Structure-Based Drug Design and Discovery of Ministry of Education, Shenyang Pharmaceutical University, Shenyang, Liaoning, 110016 China
Wuya College of Innovation, Shenyang Pharmaceutical University, Shenyang, Liaoning, 110016 China
Institute of Drug Research in Medicine Capital of China, Benxi, Liaoning, 117000 China
These authors contributed equally to this work.
Search for more papers by this authorLu Yang
Key Laboratory of Structure-Based Drug Design and Discovery of Ministry of Education, Shenyang Pharmaceutical University, Shenyang, Liaoning, 110016 China
Wuya College of Innovation, Shenyang Pharmaceutical University, Shenyang, Liaoning, 110016 China
Institute of Drug Research in Medicine Capital of China, Benxi, Liaoning, 117000 China
These authors contributed equally to this work.
Search for more papers by this authorKaizong Yao
Key Laboratory of Structure-Based Drug Design and Discovery of Ministry of Education, Shenyang Pharmaceutical University, Shenyang, Liaoning, 110016 China
Wuya College of Innovation, Shenyang Pharmaceutical University, Shenyang, Liaoning, 110016 China
Institute of Drug Research in Medicine Capital of China, Benxi, Liaoning, 117000 China
Search for more papers by this authorSen Zhang
Key Laboratory of Structure-Based Drug Design and Discovery of Ministry of Education, Shenyang Pharmaceutical University, Shenyang, Liaoning, 110016 China
Wuya College of Innovation, Shenyang Pharmaceutical University, Shenyang, Liaoning, 110016 China
Institute of Drug Research in Medicine Capital of China, Benxi, Liaoning, 117000 China
Search for more papers by this authorJiayue Fu
Key Laboratory of Structure-Based Drug Design and Discovery of Ministry of Education, Shenyang Pharmaceutical University, Shenyang, Liaoning, 110016 China
Wuya College of Innovation, Shenyang Pharmaceutical University, Shenyang, Liaoning, 110016 China
Institute of Drug Research in Medicine Capital of China, Benxi, Liaoning, 117000 China
Search for more papers by this authorHanyang Sun
Key Laboratory of Structure-Based Drug Design and Discovery of Ministry of Education, Shenyang Pharmaceutical University, Shenyang, Liaoning, 110016 China
Wuya College of Innovation, Shenyang Pharmaceutical University, Shenyang, Liaoning, 110016 China
Institute of Drug Research in Medicine Capital of China, Benxi, Liaoning, 117000 China
Search for more papers by this authorHongsheng Xu
Zhejiang Shapuaisi Pharmaceutical Co., Ltd., Pinghu, Zhejiang, 314299 China
Search for more papers by this authorBin Lin
Key Laboratory of Structure-Based Drug Design and Discovery of Ministry of Education, Shenyang Pharmaceutical University, Shenyang, Liaoning, 110016 China
Wuya College of Innovation, Shenyang Pharmaceutical University, Shenyang, Liaoning, 110016 China
Institute of Drug Research in Medicine Capital of China, Benxi, Liaoning, 117000 China
Search for more papers by this authorMaosheng Cheng
Key Laboratory of Structure-Based Drug Design and Discovery of Ministry of Education, Shenyang Pharmaceutical University, Shenyang, Liaoning, 110016 China
Institute of Drug Research in Medicine Capital of China, Benxi, Liaoning, 117000 China
Search for more papers by this authorCorresponding Author
Yongxiang Liu
Key Laboratory of Structure-Based Drug Design and Discovery of Ministry of Education, Shenyang Pharmaceutical University, Shenyang, Liaoning, 110016 China
Wuya College of Innovation, Shenyang Pharmaceutical University, Shenyang, Liaoning, 110016 China
Institute of Drug Research in Medicine Capital of China, Benxi, Liaoning, 117000 China
E-mail: [email protected]Search for more papers by this authorComprehensive Summary
An asymmetric synthesis of dihydrospirotryprostatin B was achieved in 15 steps (8 purifications) from L-tryptophan. The main feature of our synthetic strategy is the efficient construction of spirocyclic oxindole intermediate containing a chiral quaternary carbon center, involving the silica gel-mediated cyclization of tryptamine-ynamide and oxidation under neat conditions.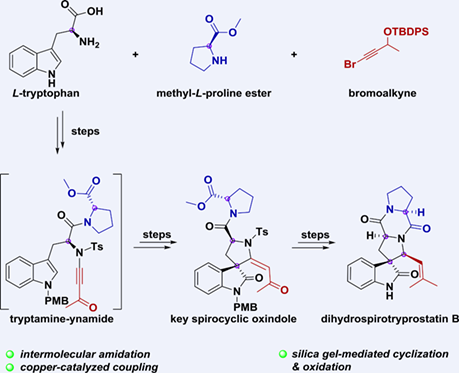
Supporting Information
| Filename | Description |
|---|---|
| cjoc202300727-sup-0001-supinfo.pdfPDF document, 1.3 MB |
Appendix S1: Supporting Information |
Please note: The publisher is not responsible for the content or functionality of any supporting information supplied by the authors. Any queries (other than missing content) should be directed to the corresponding author for the article.
References
- 1(a) Cui, C. B.; Kakeya, H.; Osada, H. Spirotryprostatin B, a Novel Mammalian Cell Cycle Inhibitor Produced by Aspergillus Fumigatus. J. Antibiot. 1996, 49, 832–835; (b) Cui, C. B.; Kakeya, H.; Okada, G.; Onose, R.; Osada, H. Novel Mammalian Cell Cycle Inhibitors, Tryprostatins A, B and Other Diketopiperazines Produced by Aspergillus Fumigates. J. Antibiot. 1996, 49, 527–533; (c) Cui, C. B.; Kakeya, H.; Osada, H. Novel Mammalian Cell Cycle Inhibitors, Spirotryprostatins A and B, Produced by Aspergillus Fumigatus, which Inhibit Mammalian Cell Cycle at G2/M Phase. Tetrahedron 1996, 52, 12651–12666.
- 2 Wang, F. Z.; Fang, Y. C.; Zhu, T. J.; Zhang, M.; Lin, A. Q.; Gu, Q. Q.; Zhu, W. M. Seven New Prenylated Indole Diketopiperazine Alkaloids from Holothurian-Derived Fungus Aspergillus Fumigatus. Tetrahedron 2008, 64, 7986–7991.
- 3 Afiyatullov, S. S.; Zhuravleva, O. I.; Chaikina, E. L.; Anisimov, M. M. A New Spirotryprostatin from the Marine Isolate of the Fungus Aspergillus Fumigatus. Chem. Nat. Compd. 2012, 48, 95–98.
- 4For selected syntheses of spirotryprostatin A and derivatives, see: (a) Edmondson, S. D.; Danishefsky, S. J. The Total Synthesis of Spirotryprostatin A. Angew. Chem. Int. Ed. 1998, 37, 1138–1140;
10.1002/(SICI)1521-3773(19980504)37:8<1138::AID-ANIE1138>3.0.CO;2-N CAS PubMed Web of Science® Google Scholar(b) Edmondson, S.; Danishefsky, S. J.; Sepp-Lorenzino, L.; Rosen, N. Total Synthesis of Spirotryprostatin A, Leading to the Discovery of Some Biologically Promising Analogues. J. Am. Chem. Soc. 1999, 121, 2147–2155; (c) Onishi, T.; Sebahar, P. R.; Williams, R. M. Concise, Asymmetric Total Synthesis of Spirotryprostatin A. Org. Lett. 2003, 5, 3135–3137; (d) Onishi, T.; Sebahar, P. R.; Williams, R. M. Concise, Asymmetric Total Synthesis of Spirotryprostatin A. Tetrahedron 2004, 60, 9503–9515; (e) Miyake, F. Y.; Yakushijin, K.; Horne, D. A. A Concise Synthesis of Spirotryprostatin A. Org. Lett. 2004, 6, 4249–4251; (f) Cheng, M. N.; Wang, H.; Gong, L. Z. Asymmetic Organocatalytic 1,3-Dipolar Cycloaddition of Azomethine Ylide to Methyl 2-(2-Nitrophenyl)acrylate for the Synthesis of Diastereoisomers of Spirotryprostatin A. Org. Lett. 2011, 13, 2418–2421; (g) Antonchick, A. P.; Schuster, H.; Bruss, H.; Schürmann, M.; Preut, H.; Rauh, D.; Waldmann, H. Enantioselective Synthesis of the Spirotryprostatin A Scaffold. Tetrahedron 2011, 67, 10195–10202; (h) Kitahara, K.; Shimokawa, J.; Fukuyama, T. Stereoselective Synthesis of Spirotryprostatin A. Chem. Sci. 2014, 5, 904–907; (i) Ma, Y. M.; Fan, C.; Jia, B.; Cheng, P.; Liu, J.; Ma, Y. Q.; Qiao, K. Total Synthesis and Biological Evaluation of Spirotryprostatin A Analogs. Chirality 2017, 29, 737–746; (j) Peng, T. F.; Liu, T.; Zhao, J. F.; Dong, J. W.; Zhao, Y. X.; Yang, Y. X.; Yan, X.; Xu, W. L.; Shen, X. F. Enantioselective Total Synthesis of Spirotryprostatin A. J. Org. Chem. 2022, 87, 16743–16754.
- 5For selected syntheses of spirotryprostatin B and derivatives, see: (a) Sebahar, P. R.; Williams, R. M. The Asymmetric Total Synthesis of (+)- and (−)-Spirotryprostatin B. J. Am. Chem. Soc. 2000, 122, 5666–5667;
(b) Von Nussbaum, F.; Danishefsky, S. J. A Rapid Total Synthesis of Spirotryprostatin B: Proof of Its Relative and Absolute Stereochemistry. Angew. Chem. Int. Ed. 2000, 39, 2175–2178;
10.1002/1521-3773(20000616)39:12<2175::AID-ANIE2175>3.0.CO;2-J CAS PubMed Web of Science® Google Scholar(c) Wang, H. S.; Ganesan, A. A Biomimetic Total Synthesis of (−)-Spirotryprostatin B and Related Studies. J. Org. Chem. 2000, 65, 4685–4693; (d) Overman, L. E.; Rosen, M. D. Total Synthesis of (−)-Spirotryprostatin B and Three Stereoisomers. Angew. Chem. Int. Ed. 2000, 39, 4596–4599;10.1002/1521-3773(20001215)39:24<4596::AID-ANIE4596>3.0.CO;2-F CAS PubMed Web of Science® Google Scholar(e) Bagul, T. D.; Lakshmaiah, G.; Kawabata, T.; Fuji, K. Total Synthesis of Spirotryprostatin B via Asymmetric Nitroolefination. Org. Lett. 2002, 4, 249–251; (f) Meyers, C.; Carreira, E. M. Total Synthesis of (−)-Spirotryprostatin B. Angew. Chem. Int. Ed. 2003, 42, 694–696; (g) Miyake, F. Y.; Yakushijin, K.; Horne, D. A. Preparation and Synthetic Applications of 2-Halotryptophan Methyl Esters: Synthesis of Spirotryprostatin B. Angew. Chem. Int. Ed. 2004, 43, 5357–5360; (h) Marti, C.; Carreira, E. M. Total Synthesis of (−)-Spirotryprostatin B: Synthesis and Related Studies. J. Am. Chem. Soc. 2005, 127, 11505–11515; (i) Trost, B. M.; Stiles, D. T. Total Synthesis of Spirotryprostatin B via Diastereoselective Prenylation. Org. Lett. 2007, 9, 2763–2766; (j) Xi, Y. K.; Zhang, H. B.; Li, R. X.; Kang, S. Y.; Li, J.; Li, Y. Total Synthesis of Spirotryprostatins through Organomediated Intramolecular Umpolung Cyclization. Chem. – Eur. J. 2019, 25, 3005–3009; (k) Song, H. Q.; Song, J. C.; Yan, L. H.; He, W. G.; Wang, P. Y.; Xu, Y. Z.; Wei, H. B.; Xie, W. Q. A Concise Synthesis of (–)-Dihydrospirotryprostatin B via Tandem Michael Addition. Tetrahedron Lett. 2021, 85, 153486.
- 6(a) Wang, Y. S.; Lin, J. S.; Wang, X. Y.; Wang, G. H.; Zhang, X. H.; Yao, B.; Zhao, Y. D.; Yu, P. F.; Lin, B.; Liu, Y. X.; Cheng, M. S. Brønsted Acid-Catalyzed Tandem Cyclizations of Tryptamine-Ynamides Yielding 1H-Pyrrolo[2,3-d]Carbazole Derivatives. Chem. – Eur. J. 2018, 24, 3913–3913; (b) Wang, Y. S.; Wang, X. Y.; Lin, J. S.; Yao, B.; Wang, G. H.; Zhao, Y. D.; Zhang, X. H.; Lin, B.; Liu, Y.; Cheng, M. S.; Liu, Y. X. Ynesulfonamide-Based Silica Gel and Alumina-Mediated Diastereoselective Cascade Cyclizations to Spiro[Indoline-3,3′-Pyrrolidin]-2-Ones under Neat Conditions. Adv. Synth. Catal. 2018, 360, 1483–1492; (c) Liu, C. J.; Sun, Z. H.; Xie, F. K.; Liang, G. D.; Yang, L.; Li, Y. Q.; Cheng, M. S.; Lin, B.; Liu, Y. X. Gold(I)-Catalyzed Pathway-Switchable Tandem Cycloisomerizations to Indolizino[8,7-b]Indole and Indolo[2,3-a]Quinolizine Derivatives. Chem. Commun. 2019, 55, 14418–14421; (d) Pang, Y. D.; Liang, G. D.; Xie, F. K.; Hu, H. B.; Du, C.; Zhang, X. H.; Cheng, M. S.; Lin, B.; Liu, Y. X. N-Fluorobenzenesulfonimide as a Highly Effective Ag(I)-Catalyst Attenuator for Tryptamine-Derived Ynesulfonamide Cycloisomerization. Org. Biomol. Chem. 2019, 17, 2247–2257; (e) Liang, G. D.; Ji, Y. J.; Liu, H. R.; Pang, Y. D.; Zhou, B. J.; Cheng, M. S.; Liu, Y.; Lin, B.; Liu, Y. X. Silver Triflate/N-Fluorobenzenesulfonimide-Catalyzed Cycloisomerization of Tryptamine-Ynamide to Spiro[Indoline-3,4′-piperidine] Induced by Cation-π-π Interactions between Substrate and Metal Ligand. Adv. Synth. Catal. 2020, 362, 192–205; (f) Liang, G. D.; Pang, Y. D.; Ji, Y. J.; Zhuang, K. T.; Li, L. J.; Xie, F. K.; Yang, L.; Cheng, M. S.; Lin, B.; Liu, Y. X. Diastereoselective Syntheses of Spiro[Indoline-3,4′-Pyridin]-2-Yl Carbamates via AgOTf/Ph3P-Catalyzed Tandem Cyclizations of Tryptamine-Ynesulfonamides. J. Org. Chem. 2020, 85, 3010–3019; (g) Chen, Y. Y.; Wang, Z. B.; Zhao, W. T.; Sun, S. T.; Yang, L.; Zhang, J. P.; Zhang, D.; Cheng, M. S.; Lin, B.; Liu, Y. X. Ag(I)/PPh3-Catalyzed Diastereoselective Syntheses of Spiro[Indole-3,4′-Piperidine] Derivatives via Cycloisomerizations of Tryptamine-Ynamides. Chem. Commun. 2022, 58, 3051–3054; (h) Yang, L.; Hou, A. B.; Jiang, Q.; Cheng, M. S.; Liu, Y. X. Methodological Development and Applications of Tryptamine-Ynamide Cyclizations in Synthesizing Core Skeletons of Indole Alkaloids. J. Org. Chem. 2023, 88, 11377–11391.
- 7 Yang, L.; Huang, S. W.; Huang, R. K.; Hou, A. B.; Zhang, S.; Su, H. W.; Ding, X. H.; Lin, B.; Cheng, M. S.; Liu, Y. X. Total Syntheses of Aspidospermidine, N-Methylaspidospermidine, N-Acetylaspidospermidine, and Aspidospermine via a Tandem Cyclization of Tryptamine-Ynamide. Org. Lett. 2021, 23, 6471–6476.
- 8 Shi, H.; Du, C.; Zhang, X. H.; Xie, F. K.; Wang, X. Y. Cui, S. S.; Peng, X. S.; Cheng, M. S.; Lin, B.; Liu, Y. X. Lewis Acid Assisted Electrophilic Fluorine-Catalyzed Pinacol Rearrangement of Hydrobenzoin Substrates: One-Pot Synthesis of (±)-Latifine and (±)-Cherylline. J. Org. Chem. 2018, 83, 1312–1319.




