1,3-Dipolar Cycloaddition of Polycyclic Aromatic Azomethine Ylides and Alkynylbenziodoxoles for Synthesis of Functional Dibenzoullazines†
Hui Han
School of Chemistry, Chemical Engineering and Biotechnology, Nanyang Technological University, 21 Nanyang Link, Singapore, 637371 Singapore
Search for more papers by this authorGlen Wee Zhuan Goh
School of Chemistry, Chemical Engineering and Biotechnology, Nanyang Technological University, 21 Nanyang Link, Singapore, 637371 Singapore
Search for more papers by this authorYongxin Li
School of Chemistry, Chemical Engineering and Biotechnology, Nanyang Technological University, 21 Nanyang Link, Singapore, 637371 Singapore
Search for more papers by this authorNaohiko Yoshikai
Graduate School of Pharmaceutical Sciences, Tohoku University, Sendai, 980-8578 Japan
Search for more papers by this authorCorresponding Author
Shingo Ito
School of Chemistry, Chemical Engineering and Biotechnology, Nanyang Technological University, 21 Nanyang Link, Singapore, 637371 Singapore
E-mail: [email protected]Search for more papers by this authorHui Han
School of Chemistry, Chemical Engineering and Biotechnology, Nanyang Technological University, 21 Nanyang Link, Singapore, 637371 Singapore
Search for more papers by this authorGlen Wee Zhuan Goh
School of Chemistry, Chemical Engineering and Biotechnology, Nanyang Technological University, 21 Nanyang Link, Singapore, 637371 Singapore
Search for more papers by this authorYongxin Li
School of Chemistry, Chemical Engineering and Biotechnology, Nanyang Technological University, 21 Nanyang Link, Singapore, 637371 Singapore
Search for more papers by this authorNaohiko Yoshikai
Graduate School of Pharmaceutical Sciences, Tohoku University, Sendai, 980-8578 Japan
Search for more papers by this authorCorresponding Author
Shingo Ito
School of Chemistry, Chemical Engineering and Biotechnology, Nanyang Technological University, 21 Nanyang Link, Singapore, 637371 Singapore
E-mail: [email protected]Search for more papers by this authorDedicated to the Special Issue of Emerging Investigators in 2023.
Comprehensive Summary
A new family of dibenzoullazine derivatives was synthesized through 1,3-dipolar cycloaddition of polycyclic aromatic azomethine ylides with alkynylbenziodoxoles followed by oxidation. The benziodoxole moiety in the resulting products was used as a versatile linchpin for the synthesis of structurally diverse functional dibenzoullazines that are difficult to access by other synthetic methods.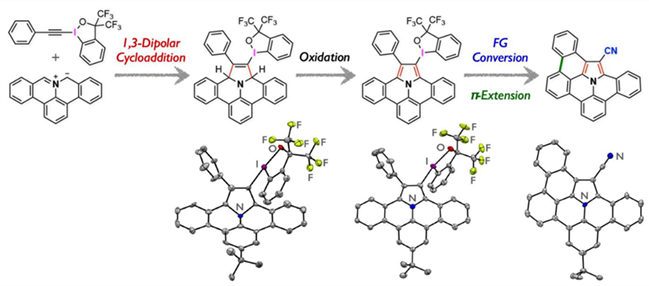
Supporting Information
| Filename | Description |
|---|---|
| cjoc202300637-sup-0001-supinfo.pdfPDF document, 3.8 MB |
Appendix S1: Supporting Information |
Please note: The publisher is not responsible for the content or functionality of any supporting information supplied by the authors. Any queries (other than missing content) should be directed to the corresponding author for the article.
References
- 1 Stępień, M.; Gońka, E.; Żyła, M.; Sprutta, N. Heterocyclic Nanographenes and Other Polycyclic Heteroaromatic Compounds: Synthetic Routes, Properties, and Applications. Chem. Rev. 2017, 117, 3479–3716.
- 2 Borissov, A.; Maurya, Y. K.; Moshniaha, L.; Wong, W.-S.; Żyła-Karwowska, M.; Stępień, M. Recent Advances in Heterocyclic Nanographenes and Other Polycyclic Heteroaromatic Compounds. Chem. Rev. 2022, 122, 565–788.
- 3 Cebrián, C. Ullazine-Based Materials: Towards Novel Opportunities in Organic Electronics. J. Mater. Chem. C 2018, 6, 11943–11950.
- 4 Delcamp, J. H.; Yella, A.; Holcombe, T. W.; Nazeeruddin, M. K.; Grätzel, M. The Molecular Engineering of Organic Sensitizers for Solar-Cell Applications. Angew. Chem. Int. Ed. 2013, 52, 376–380.
- 5 Dualeh, A.; Humphry-Baker, R.; Delcamp, J. H.; Nazeeruddin, M. K.; Grätzel, M. Solid-State Dye-Sensitized Solar Cells Using a Novel Class of Ullazine Dyes as Sensitizers. Adv. Energy Mater. 2013, 3, 496–504.
- 6 Qiao, H.; Deng, Y.; Peng, R.; Wang, G.; Yuan, J.; Tan, S. Effect of π-Spacers and Anchoring Groups on the Photovoltaic Performances of Ullazine-Based Dyes. RSC Adv. 2016, 6, 70046–70055.
- 7 Zhang, Y.; Cheema, H.; McNamara, L.; Hunt, L. A.; Hammer, N. I.; Delcamp, J. H. Ullazine Donor–π Bridge-Acceptor Organic Dyes for Dye-Sensitized Solar Cells. Chem. Eur. J. 2018, 24, 5939–5949.
- 8 Xia, J.; Cavazzini, M.; Igci, C.; Momblona, C.; Orlandi, S.; Ding, B.; Zhang, Y.; Kanda, H.; Klipfel, N.; Khan, S. B.; Asiri, A. M.; Dyson, P. J.; Pozzi, G.; Nazeeruddin, M. K. Molecular Engineering of Thienyl Functionalized Ullazines as Hole-Transporting Materials for Perovskite Solar Cells. Sol. RRL 2022, 6, 2100926.
- 9 Skabeev, A.; Zschieschang, U.; Zagranyarski, Y.; Klauk, H.; Müllen, K.; Li, C. Carbonyl-Functionalized Cyclazines as Colorants and Air-Stable n-Type Semiconductors. Org. Lett. 2018, 20, 1409−1412.
- 10 Dumele, O.; Đorđević, L.; Sai, H.; Cotey, T. J.; Sangji, M. H.; Sato, K.; Dannenhoffer, A. J.; Stupp, S. I. Photocatalytic Aqueous CO2 Reduction to CO and CH4 Sensitized by Ullazine Supramolecular Polymers. J. Am. Chem. Soc. 2022, 144, 3127–3136.
- 11 Das, A.; Ghosh, I.; König, B. Synthesis of Pyrrolo[1,2-a]quinolines and Ullazines by Visible Light Mediated One- and Twofold Annulation of N-Arylpyrroles with Arylalkynes. Chem. Commun. 2016, 52, 8695–8698.
- 12 Zhang, G.; Gautama, P.; Chan, J. M. W. Symmetrical and Unsymmetrical Fluorine-Rich Ullazines via Controlled Cycloaromatizations. Org. Chem. Front. 2020, 7, 787–795.
- 13 Kanno, K.; Liu, Y.; Iesato, A.; Nakajima, K.; Takahashi, T. Chromium-Mediated Synthesis of Polycyclic Aromatic Compounds from Halobiaryls. Org. Lett. 2005, 7, 5453–5456.
- 14 Wan, D.; Li, X.; Jiang, R.; Feng, B.; Lan, J.; Wang, R.; You, J. Palladium-Catalyzed Annulation of Internal Alkynes: Direct Access to π-Conjugated Ullazines. Org. Lett. 2016, 18, 2876−2879.
- 15 Hu, Y.; Jia, Y.; Tuo, Z.; Zhou, W. Rhodium(III)-Catalyzed Intramolecular Annulation and Aromatization for the Synthesis of Pyrrolo[1,2-a]quinolines. Org. Lett. 2023, 25, 1845–1849.
- 16 Zhou, J.; Yang, W.; Wang, B.; Ren, H. Friedel–Crafts Arylation for the Formation of Csp2–Csp2 Bonds: A Route to Unsymmetrical and Functionalized Polycyclic Aromatic Hydrocarbons from Aryl Triazenes. Angew. Chem. Int. Ed. 2012, 51, 12293–12297.
- 17 Miao, D.; Aumaitre, C.; Morin, J.-F. Photochemical Synthesis of π-Extended Ullazine Derivatives as New Electron Donors for Efficient Conjugated D–A Polymers. J. Mater. Chem. C 2019, 7, 3015–3024.
- 18 Hager, J.; Kang, S.; Chmielewski, P. J.; Lis, T.; Kim, D.; Stępień, M. Acenaphthylene-Fused Ullazines: Fluorescent π-Extended Monopyrroles with Tunable Electronic Gaps. Org. Chem. Front. 2022, 9, 3179–3185.
- 19 Wang, D.; Liu, Y.; Wang, L.; Cheng, H.; Zhang, Y.; Gao, G. Synthesis of π-Extended Dibenzo[d,k]ullazines by a Palladium-Catalyzed Double Annulation Using Arynes. Chin. Chem. Lett. 2021, 32, 1407–1410.
- 20 Ito, S. The Rapid Synthesis of π-Extended Azacorannulenes. J. Synth. Org. Chem. Jpn. 2019, 77, 1128–1135.
- 21 Ito, S.; Tokimaru, Y.; Nozaki, K. Isoquinolino[4,3,2-de]phenanthridine: Synthesis and Its Use in 1,3-Dipolar Cycloadditions to Form Nitrogen-Containing Polyaromatic Hydrocarbons. Chem. Commun. 2015, 51, 221–224.
- 22 Tokimaru, Y.; Ito, S.; Nozaki, K. Synthesis of Pyrrole-Fused Corannulenes: 1,3-Dipolar Cycloaddition of Azomethine Ylides to Corannulene. Angew. Chem. Int. Ed. 2017, 56, 15560–15564.
- 23 Tokimaru, Y.; Ito, S.; Nozaki, K. A Hybrid of Corannulene and Azacorannulene: Synthesis of a Highly Curved Nitrogen-Containing Buckybowl. Angew. Chem. Int. Ed. 2018, 57, 9818–9822.
- 24 Nakamura, K.; Li, Q.-Q.; Krejčí, O.; Foster, A. S.; Sun, K.; Kawai, S.; Ito, S. On-Surface Synthesis of a π-Extended Diaza[8]circulene. J. Am. Chem. Soc. 2020, 142, 11363–11369.
- 25 Zhang, X.; Mackinnon, M. R.; Bodwell, G. J.; Ito, S. Synthesis of a π-Extended Azacorannulenophane Enabled by Strain-Induced 1,3-Dipolar Cycloaddition. Angew. Chem. Int. Ed. 2022, 61, e202116585.
- 26 Wang, W.; Hanindita, F.; Tanaka, Y.; Ochiai, K.; Sato, H.; Li, Y.; Yasuda, T.; Ito, S. π-Extended Pyrrole-Fused Heteropine: Synthesis, Properties, and Application in Organic Field-Effect Transistors. Angew. Chem. Int. Ed. 2023, 62, e202218176.
- 27 Ito, S.; Tokimaru, Y.; Nozaki, K. Benzene-Fused Azacorannulene Bearing an Internal Nitrogen Atom. Angew. Chem. Int. Ed. 2015, 54, 7256–7260.
- 28 Wang, W.; Hanindita, F.; Hamamoto, Y.; Li, Y.; Ito, S. Fully Conjugated Azacorannulene Dimer as Large Diaza[80]fullerene Fragment. Nat. Commun. 2022, 13, 1498.
- 29 Nakamura, K.; Ochiai, K.; Yubuta, A.; He, D.; Miyajima, D.; Ito, S. Pyridine-Fused Azacorannulene: Fine-Tuning of the Structure and Properties of Nitrogen-Embedded Buckybowls. Precis. Chem. 2023, 1, 29–33.
- 30 Berger, R.; Wagner, M.; Feng, X.; Müllen, K. Polycyclic Aromatic Azomethine Ylides: A Unique Entry to Extended Polycyclic Heteroaromatics. Chem. Sci. 2015, 6, 436–441.
- 31 Richter, M.; Hahn, S.; Dmitrieva, E.; Rominger, F.; Popov, A.; Bunz, U. H. F.; Feng, X.; Berger, R. Helical Ullazine-Quinoxaline-Based Polycyclic Aromatic Hydrocarbons. Chem. Eur. J. 2019, 25, 1345–1352.
- 32 Richter, M.; Fu, Y.; Dmitrieva, E.; Weigand, J. J.; Popov, A.; Berger, R.; Liu, J.; Feng, X. Polycyclic Aromatic Hydrocarbons Containing a Pyrrolopyridazine Core. ChemPlusChem 2019, 84, 613–618.
- 33 Skidin, D.; Eisenhut, F.; Richter, M.; Nikipar, S.; Krüger, J.; Ryndyk, D. A.; Berger, R.; Cuniberti, G.; Feng, X.; Moresco, F. On-Surface Synthesis of Nitrogen-Doped Nanographenes with 5–7 Membered Rings. Chem. Commun. 2019, 55, 4731–4734.
- 34 Li, S.; Sun, Y.; Li, X.; Smaga, O.; Koniarz, S.; Stępień, M.; Chmielewski, P. J. 1,3-Dipolar Cycloaddition of Polycyclic Azomethine Ylide to Norcorroles: Towards Dibenzoullazine-fused Derivatives. Chem. Commun. 2022, 58, 6510–6513.
- 35 Yoshimura, A.; Zhdankin, V. V. Advances in Synthetic Applications of Hypervalent Iodine Compounds. Chem. Rev. 2016, 116, 3328–3435.
- 36 Mironova, I. A.; Noskov, D. M.; Yoshimura, A.; Yusubov, M. S.; Zhdankin, V. V. Aryl-, Alkynyl-, and Alkenylbenziodoxoles: Synthesis and Synthetic Applications. Molecules 2023, 28, 2136.
- 37 Le Du, E.; Waser, J. Recent Progress in Alkynylation with Hypervalent Iodine Reagents. Chem. Commun. 2023, 59, 1589–1604.
- 38 Stang, P. J.; Murch, P. [3+2]-Cycloaddition Reactions of Alkynyl(phenyl)iodonium Triflates with Ethyl Diazoacetate, N-t-Butyl-α-phenyl Nitrone and t-Butylnitrileoxide as 1,3-Dipoles. Tetrahedron 1997, 38, 8793–8794.
- 39 Kitamura, T.; Mansei, Y.; Fujiwara, Y. 1,3-Dipolar Cycloaddition of Alkynyliodonium Salts with a Nitrile Oxide. Synthesis and Reactivity of Isoxazolyliodonium Salts. J. Organomet. Chem. 2002, 646, 196–199.
- 40 Wu, B.; Wu, J.; Yoshikai, N. Benziodoxole Triflate as a Versatile Reagent for Iodo(III)cyclization of Alkynes. Chem. Asian J. 2017, 12, 3123–3127.
- 41
Ding, W.; Chai, J.; Wang, C.; Wu, J.; Yoshikai, N. Stereoselective Access to Highly Substituted Vinyl Ethers via trans-Difunctionalization of Alkynes with Alcohols and Iodine(III) Electrophile. J. Am. Chem. Soc. 2020, 14, 8619–8624.
10.1021/jacs.0c04140 Google Scholar
- 42 Ding, W.; Wang, C.; Tan, J. R.; Ho, C. C.; León, F.; García, F.; Yoshikai, N. Site-Selective Aromatic C–H λ3-Iodanation with a Cyclic Iodine(III) Electrophile in Solution and Solid Phases. Chem. Sci. 2020, 11, 7356–7361.
- 43 Wang, C.-S.; Tan, P. S. L.; Ding, W.; Ito, S.; Yoshikai, N. Regio- and Stereoselective Synthesis of Enol Carboxylate, Phosphate, and Sulfonate Esters via Iodo(III)functionalization of Alkynes. Org. Lett. 2022, 24, 430–434.




