Nucleophilic Substitution Polymerization-Induced Emission of 1,3-Dicarbonyl Compounds as a Versatile Approach for Aggregation-Induced Emission Type Non-Traditional Intrinsic Luminescence†
Yu-Jiao Chen
College of Environment and Resources, Engineering Research Center of Polymer Green Recycling of Ministry of Education, Fujian Key Laboratory of Pollution Control &Resource Reuse, Fujian Normal University, Fuzhou, Fujian, 350007 China
Key Laboratory of Coal to Ethylene Glycol and Its Related Technology, State Key Laboratory of Structural Chemistry, Center for Excellence in Molecular Synthesis, Fujian Institute of Research on the Structure of Matter, Chinese Academy of Sciences, 155 Yangqiao Road West, Fuzhou, Fujian, 350002 China
Search for more papers by this authorMeng-Qin Pu
Key Laboratory of Coal to Ethylene Glycol and Its Related Technology, State Key Laboratory of Structural Chemistry, Center for Excellence in Molecular Synthesis, Fujian Institute of Research on the Structure of Matter, Chinese Academy of Sciences, 155 Yangqiao Road West, Fuzhou, Fujian, 350002 China
College of Chemistry and Materials Science, Fujian Normal University, Fuzhou, Fujian, 350007 China
Search for more papers by this authorLiang-Tao Wu
College of Environment and Resources, Engineering Research Center of Polymer Green Recycling of Ministry of Education, Fujian Key Laboratory of Pollution Control &Resource Reuse, Fujian Normal University, Fuzhou, Fujian, 350007 China
Key Laboratory of Coal to Ethylene Glycol and Its Related Technology, State Key Laboratory of Structural Chemistry, Center for Excellence in Molecular Synthesis, Fujian Institute of Research on the Structure of Matter, Chinese Academy of Sciences, 155 Yangqiao Road West, Fuzhou, Fujian, 350002 China
Search for more papers by this authorCorresponding Author
Xiao-Li Sun
College of Environment and Resources, Engineering Research Center of Polymer Green Recycling of Ministry of Education, Fujian Key Laboratory of Pollution Control &Resource Reuse, Fujian Normal University, Fuzhou, Fujian, 350007 China
E-mail: [email protected]; [email protected]Search for more papers by this authorCorresponding Author
Wen-Ming Wan
Key Laboratory of Coal to Ethylene Glycol and Its Related Technology, State Key Laboratory of Structural Chemistry, Center for Excellence in Molecular Synthesis, Fujian Institute of Research on the Structure of Matter, Chinese Academy of Sciences, 155 Yangqiao Road West, Fuzhou, Fujian, 350002 China
College of Chemistry and Materials Science, Fujian Normal University, Fuzhou, Fujian, 350007 China
E-mail: [email protected]; [email protected]Search for more papers by this authorYu-Jiao Chen
College of Environment and Resources, Engineering Research Center of Polymer Green Recycling of Ministry of Education, Fujian Key Laboratory of Pollution Control &Resource Reuse, Fujian Normal University, Fuzhou, Fujian, 350007 China
Key Laboratory of Coal to Ethylene Glycol and Its Related Technology, State Key Laboratory of Structural Chemistry, Center for Excellence in Molecular Synthesis, Fujian Institute of Research on the Structure of Matter, Chinese Academy of Sciences, 155 Yangqiao Road West, Fuzhou, Fujian, 350002 China
Search for more papers by this authorMeng-Qin Pu
Key Laboratory of Coal to Ethylene Glycol and Its Related Technology, State Key Laboratory of Structural Chemistry, Center for Excellence in Molecular Synthesis, Fujian Institute of Research on the Structure of Matter, Chinese Academy of Sciences, 155 Yangqiao Road West, Fuzhou, Fujian, 350002 China
College of Chemistry and Materials Science, Fujian Normal University, Fuzhou, Fujian, 350007 China
Search for more papers by this authorLiang-Tao Wu
College of Environment and Resources, Engineering Research Center of Polymer Green Recycling of Ministry of Education, Fujian Key Laboratory of Pollution Control &Resource Reuse, Fujian Normal University, Fuzhou, Fujian, 350007 China
Key Laboratory of Coal to Ethylene Glycol and Its Related Technology, State Key Laboratory of Structural Chemistry, Center for Excellence in Molecular Synthesis, Fujian Institute of Research on the Structure of Matter, Chinese Academy of Sciences, 155 Yangqiao Road West, Fuzhou, Fujian, 350002 China
Search for more papers by this authorCorresponding Author
Xiao-Li Sun
College of Environment and Resources, Engineering Research Center of Polymer Green Recycling of Ministry of Education, Fujian Key Laboratory of Pollution Control &Resource Reuse, Fujian Normal University, Fuzhou, Fujian, 350007 China
E-mail: [email protected]; [email protected]Search for more papers by this authorCorresponding Author
Wen-Ming Wan
Key Laboratory of Coal to Ethylene Glycol and Its Related Technology, State Key Laboratory of Structural Chemistry, Center for Excellence in Molecular Synthesis, Fujian Institute of Research on the Structure of Matter, Chinese Academy of Sciences, 155 Yangqiao Road West, Fuzhou, Fujian, 350002 China
College of Chemistry and Materials Science, Fujian Normal University, Fuzhou, Fujian, 350007 China
E-mail: [email protected]; [email protected]Search for more papers by this authorDedicated to the Special Issue of Emerging Themes in Polymer Science.
Comprehensive Summary
Nucleophilic substitution reaction and 1,3-dicarbonyl compounds play significant roles in organic chemistry, and non-traditional intrinsic luminescence (NTIL) has become an emerging research area. Here, we demonstrate the successful nucleophilic substitution polymerization of 1,3-dicarbonyl compounds, including acetylacetone, 3,5-heptanedione, methyl acetoacetate, cyclopentane-1,3-dione, 1,3-indandione, 1-phenyl-1,3-butanedione and dibenzoylmethane, where reactive hydrogens at α position of 1,3-dicarbonyl compounds are involved. Through this base catalyzed nucleophilic substitution polycondensation between 1,3-dicarbonyl compounds and α,α’-dibromo xylene, a series of nonconjugated poly(1,3-dicarbonyl)s have been successfully prepared with high yield (up to >99%) under mild conditions. Investigations reveal that this nucleophilic substitution polycondensation exhibits self-accelerating effect and flexible stoichiometry characteristics, which exhibits advantages over traditional polycondensation methods. This polymerization also exhibits intriguing polymerization-induced emission (PIE) characteristics, where nonconjugated poly(1,3-dicarbonyl)s exhibit intriguing chemical structure dependent aggregation-induced emission (AIE) type NTIL. This work therefore expands the monomer, method, chemical structure and property libraries of polymer chemistry, which may cause inspirations to polymerization methodology, PIE, AIE and NTIL.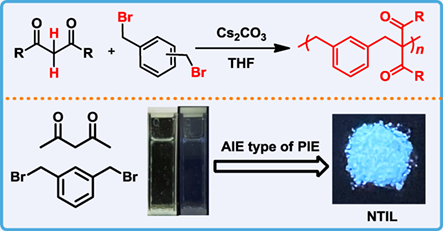
Supporting Information
| Filename | Description |
|---|---|
| cjoc202200794-sup-0001-supinfo.pdfPDF document, 1.5 MB |
Appendix S1: Supporting Information |
Please note: The publisher is not responsible for the content or functionality of any supporting information supplied by the authors. Any queries (other than missing content) should be directed to the corresponding author for the article.
References
- 1 Weng, C.; Fan, N. Q.; Xu, T. R.; Chen, H. D.; Li, Z. F.; Li, Y. W.; Tan, H.; Fu, Q.; Ding, M. M. FRET-based polymer materials for detection of cellular microenvironments. Chin. Chem. Lett. 2020, 31, 1490–1498.
- 2 Tomalia, D. A.; Klajnert-Maculewicz, B.; Johnson, K. A. M.; Brinkman, H. F.; Janaszewska, A.; Hedstrand, D. M. Non-traditional intrinsic luminescence: inexplicable blue fluorescence observed for dendrimers, macromolecules and small molecular structures lacking traditional/conventional luminophores. Prog. Polym. Sci. 2019, 90, 35–117.
- 3 Chu, B.; Zhang, H. K.; Hu, L. F.; Liu, B.; Zhang, C. J.; Zhang, X. H.; Tang, B. Z. Altering Chain Flexibility of Aliphatic Polyesters for Yellow-Green Clusteroluminescence in 38% Quantum Yield. Angew. Chem. Int. Ed. 2022, 61, e202114117.
- 4 Zhou, Q.; Cao, B. Y.; Zhu, C. X.; Xu, S.; Gong, Y. Y.; Yuan, W. Z.; Zhang, Y. M. Clustering-Triggered Emission of Nonconjugated Polyacrylonitrile. Small 2016, 12, 6586–6592.
- 5 Zhang, H. K.; Zhao, Z.; McGonigal, P. R.; Ye, R. Q.; Liu, S. J.; Lam, J. W. Y.; Kwok, R. T. K.; Yuan, W. Z.; Xie, J. P.; Rogach, A. L.; Tang, B. Z. Clusterization-triggered emission: Uncommon luminescence from common materials. Mater. Today 2020, 32, 275–292.
- 6 Hoffmann, R. Interaction of orbitals through space and through bonds. Acc. Chem. Res. 1971, 4, 1–9.
- 7 Liu, L.; Zhang, H. K.; Liu, S. J.; Sun, J. Z.; Zhang, X. H.; Tang, B. Z. Polymerization-induced emission. Mater. Horiz. 2020, 7, 987–998.
- 8 Liu, W. B.; Xu, X. H.; Kang, S. M.; Song, X.; Zhou, L.; Liu, N.; Wu, Z. Q. Bottlebrush Polymers Carrying Side Chains on Every Backbone Atom: Controlled Synthesis, Polymerization-Induced Emission, and Circularly Polarized Luminescence. Macromolecules 2021, 54, 3158–3168.
- 9 Yan, J. J.; Wang, X. Y.; Xiong, J. N.; Wang, L. Z.; Pan, D. H.; Xu, Y. P.; Yang, M. Uncovering divergent fluorescence of aliphatic polyamides: Synthesis, dual polymerization-induced emissions, and organelle- specific imaging. Chem. Eng. J. 2022, 428, 132142.
- 10 Zheng, X.; Zhang, Y. F.; Gao, L.; Wang, Z. H.; Wang, C.; Zheng, Y.; Chen, X. H.; Yang, Y.; Peng, J. X.; Qu, L. J.; Yang, C. L. Polymerization-Induced Emission of Color-Tunable Room Temperature Phosphorescence. Adv. Mater. Interfaces 2022, 9, 2200344.
- 11 Ye, S. Y.; Tian, T.; Christofferson, A. J.; Erikson, S.; Jagielski, J.; Luo, Z.; Kumar, S.; Shih, C. J.; Leroux, J. C.; Bao, Y. Y. Continuous color tuning of single-fluorophore emission via polymerization-mediated through- space charge transfer. Sci. Adv. 2021, 7, eabd1794.
- 12 Jing, Y. N.; Li, S. S.; Su, M. Q.; Bao, H. L.; Wan, W. M. Barbier Hyperbranching Polymerization-Induced Emission toward Facile Fabrication of White Light-Emitting Diode and Light-Harvesting Film. J. Am. Chem. Soc. 2019, 141, 16839–16848.
- 13 Chen, F.; Hu, J.; Wang, X. D.; Shao, S. Y.; Wang, L. X.; Jing, X. B.; Wang, F. S. Through-space charge transfer blue polymers containing acridan donor and oxygen-bridged triphenylboron acceptor for highly efficient solution-processed organic light-emitting diodes. Sci. China- Chem. 2020, 63, 1112–1120.
- 14 Tang, S. X.; Yang, T. J.; Zhao, Z. H.; Zhu, T. W.; Zhang, Q.; Hou, W. B. W.; Yuan, W. Z. Nonconventional luminophores: characteristics, advancements and perspectives. Chem. Soc. Rev. 2021, 50, 12616–12655.
- 15 Liu, B.; Chu, B.; Zhu, L. X.; Zhang, H. K.; Yuan, W. Z.; Zhao, Z.; Wan, W. M.; Zhang, X. H. Clusteroluminescence: A gauge of molecular interaction. Chin. Chem. Lett. 2023, DOI: https://doi.org/10.1016/j.cclet.2022.107909.
- 16 Ding, L.; Ding, Y. Q.; Teng, Q. W.; Wang, K. Electronic structures and spectroscopy of luminescent para-phenylenevinylene oligomers. Chin. J. Chem. 2008, 26, 97–100.
- 17 Shi, Q. X.; Li, Q.; Xiao, H.; Sun, X. L.; Bao, H. L.; Wan, W. M. Room- temperature Barbier single-atom polymerization induced emission as a versatile approach for the utilization of monofunctional carboxylic acid resources. Polym. Chem. 2022, 13, 592–599.
- 18 Shi, Q. X.; Xiao, H.; Sheng, Y. J.; Li, D. S.; Su, M.; Sun, X. L.; Bao, H. L.; Wan, W. M. Barbier single-atom polymerization induced emission as a one-pot approach towards stimuli-responsive luminescent polymers. Polym. Chem. 2022, 13, 4524–4534.
- 19 Li, D. S.; Sheng, Y. J.; Wu, L. T.; Chen, Y. J.; Xiao, H.; Sun, X. L.; Bao, H. L.; Wan, W. M. Barbier polymerization induced emission of cinnamaldehyde: a one-pot Grignard reaction? Chem. Commun. 2022, 58, 13361–13364.
- 20 Han, T.; Yan, D. Y.; Wu, Q.; Song, N.; Zhang, H. K.; Wang, D. Aggregation-Induced Emission: A Rising Star in Chemistry and Materials Science. Chin. J. Chem. 2021, 39, 677–689.
- 21 Staudinger, H. Concerning polymerisation. Ber. Dtsch. Chem. Ges. 1920, 53, 1073–1085.
- 22 Szwarc, M. Living Polymers. Nature 1956, 178, 1168–1169.
- 23 Sun, X. L.; Liu, D. M.; Pei, S.; Li, K. K.; Wan, W. M. Versatile Method to Expand the Morphology Library of Block Copolymer Solution Self-Assemblies with Tubular Structures. ACS Macro Lett. 2016, 5, 1180–1184.
- 24 Shen, D. Z.; Pan, Z. X.; Xu, H. B.; Cheng, S.; Zhu, X. L.; Fan, L. J. Synthesis, Photophysics and Morphology of a Conjugated Polymer with Azobenzene Building Block in the Backbone. Chin. J. Chem. 2010, 28, 1279–1283.
- 25 Flory, P. J. Molecular size distribution in ethylene oxide polymers. J. Am. Chem. Soc. 1940, 62, 1561–1565.
- 26 Frechet, J. M. J.; Henmi, M.; Gitsov, I.; Aoshima, S.; Leduc, M. R.; Grubbs, R. B. Self-Condensing Vinyl Polymerization - an approach to Dendritic Materials. Science 1995, 269, 1080–1083.
- 27
Kolb, H. C.; Finn, M. G.; Sharpless, K. B. Click chemistry: Diverse chemical function from a few good reactions. Angew. Chem. Int. Ed. 2001, 40, 2004–2021.
10.1002/1521-3773(20010601)40:11<2004::AID-ANIE2004>3.0.CO;2-5 CAS PubMed Web of Science® Google Scholar
- 28 Schluter, A. D. The tenth anniversary of Suzuki polycondensation (SPC). J. Polym. Sci. Part A: Polym. Chem. 2001, 39, 1533–1556.
- 29 Choi, T. L.; Grubbs, R. H. Controlled living ring-opening-metathesis polymerization by a fast-initiating ruthenium catalyst. Angew. Chem. Int. Ed. 2003, 42, 1743–1746.
- 30 Wang, D.; Liu, Y.; Hu, Z. C.; Hong, C. Y.; Pan, C. Y. Michael addition polymerizations of trifunctional amines with diacrylamides. Polymer 2005, 46, 3507–3514.
- 31 Kreye, O.; Toth, T.; Meier, M. A. R. Introducing Multicomponent Reactions to Polymer Science: Passerini Reactions of Renewable Monomers. J. Am. Chem. Soc. 2011, 133, 1790–1792.
- 32 Zheng, X. F.; Yue, M.; Yang, P.; Li, Q.; Yang, W. T. Cycloketyl radical mediated living polymerization. Polym. Chem. 2012, 3, 1982–1986.
- 33 Xue, H. D.; Zhao, Y.; Wu, H. B.; Wang, Z. L.; Yang, B.; Wei, Y.; Wang, Z. M.; Tao, L. Multicomponent Combinatorial Polymerization via the Biginelli Reaction. J. Am. Chem. Soc. 2016, 138, 8690–8693.
- 34 Wei, B.; Li, W. Z.; Zhao, Z. J.; Qin, A. J.; Hu, R. R.; Tang, B. Z. Metal-Free Multicomponent Tandem Polymerizations of Alkynes, Amines, and Formaldehyde toward Structure- and Sequence-Controlled Luminescent Polyheterocycles. J. Am. Chem. Soc. 2017, 139, 5075–5084.
- 35 Wan, W. M.; Li, S. S.; Liu, D. M.; Lv, X. H.; Sun, X. L. Synthesis of Electron-Deficient Borinic Acid Polymers with Multiresponsive Properties and Their Application in the Fluorescence Detection of Alizarin Red S and Electron-Rich 8-Hydroxyquinoline and Fluoride Ion: Substituent Effects. Macromolecules 2017, 50, 6872–6879.
- 36 Huang, H. C.; Wang, W. Q.; Zhou, Z. F.; Sun, B. H.; An, M. R.; Haeffner, F.; Niu, J. Radical Ring-Closing/Ring-Opening Cascade Polymerization. J. Am. Chem. Soc. 2019, 141, 12493–12497.
- 37 Sun, X. L.; Liu, D. M.; Wang, P.; Tan, J. L.; Li, K. K.; Deng, L.; Wan, W. M. Expanding the morphology library of block copolymer self-assemblies with clews of tubules. Chem. Commun. 2017, 53, 5005–5008.
- 38 Li, S. S.; Zhu, N. B.; Jing, Y. N.; Li, Y. J.; Bao, H. L.; Wan, W. M. Barbier Self-Condensing Ketyl Polymerization-Induced Emission: A Polarity Reversal Approach to Reversed Polymerizability. iScience 2020, 23, 101031.
- 39
Li, S. S.; Jing, Y. N.; Bao, H. L.; Wan, W. M. Exploitation of Monofunctional Carbonyl Resources by Barbier Polymerization for Materials with Polymerization-Induced Emission. Cell Rep. Phys. Sci. 2020, 1, 100116.
10.1016/j.xcrp.2020.100116 Google Scholar
- 40 Su, M.; Li, T.; Shi, Q. X.; Xiao, H.; Bao, H. L.; Wan, W. M. Barbier-Type Nitro/Nitroso Addition Polymerization as a Versatile Approach for Molecular Design of Polyarylamines through C-N Bond Formation. Macromolecules 2021, 54, 9919–9926.
- 41 Su, M.; Sheng, Y. J.; Chen, Y. J.; Li, T.; Shi, Q. X.; Xiao, H.; Pu, M. Q.; Bao, H. L.; Wan, W. M. Living Covalent-Anionic-Radical Polymerization via a Barbier Strategy. ACS Macro Lett. 2022, 11, 354–361.
- 42 Sheng, Y. J.; Su, M.; Xiao, H.; Shi, Q. X.; Sun, X. L.; Zhang, R. L.; Bao, H. L.; Wan, W. M. Barbier Hyperbranching Polymerization-Induced Emission from an AB-Type Monomer. Chem. - Eur. J. 2022, 28, e202201194.
- 43 Yang, W.; Pan, C. Y. Synthesis and Fluorescent Properties of Biodegradable Hyperbranched Poly(amido amine)s. Macromol. Rapid Commun. 2009, 30, 2096–2101.
- 44 Su, M.; Pu, M. Q.; Xiao, H.; Chen, Y. J.; Li, T.; Shi, Q. X.; Sheng, Y. J.; Bao, H. L.; Wan, W. M. Turbo-Grignard Reagent Mediated Polymerization of Styrene under Mild Conditions Capable of Low ? and Reactive Hydrogen Compatibility. Macromolecules 2022, 55, 10422–10429.
- 45 Zhang, Z. H.; Wang, Y. L.; Yan, F. F.; Peng, D. L.; Ma, Z. Photosensitive Azopolymer Brushes via Atom Transfer Radical Polymerization for Protein Sensing. Chin. J. Chem. 2011, 29, 153–158.
- 46 Pan, M. G.; Wan, D. C.; Huang, J. L. Preparation of a Novel Copolymer of Hyperbranched Polyglycerol with Multi-arms of Poly(N-isopropylacrylamide). Chin. J. Chem. 2010, 28, 499–503.
- 47 Lindley, J. Copper Assisted Nucleophilic-Substitution of Aryl Halogen. Tetrahedron 1984, 40, 1433–1456.
- 48 Bunnett, J. F.; Zahler, R. E. Aromatic Nucleophilic Substitution Reactions. Chem. Rev. 1951, 49, 273–412.
- 49 Rossi, R. A.; Pierini, A. B.; Penenory, A. B. Nucleophilic substitution reactions by electron transfer. Chem. Rev. 2003, 103, 71–167.
- 50 Lanke, V.; Marek, I. Stereospecific nucleophilic substitution at tertiary and quaternary stereocentres. Chem. Sci. 2020, 11, 9378–9385.
- 51 Johnson, R. N.; Farnham, A. G.; Clendinning, R. A.; Hale, W. F.; Merriam, C. N. Poly (Aryl Ethers) by Nucleophilic Aromatic Substitution. I. Synthesis and Rpoperties. J. Polym. Sci. Part A: Polym. Chem. 1967, 5, 2375–2398.
- 52 Yokozawa, T.; Suzuki, Y.; Hiraoka, S. Aromatic polyethers with low polydispersities from chain-growth polycondensation. J. Am. Chem. Soc. 2001, 123, 9902–9903.
- 53 Yokozawa, T.; Ogawa, M.; Sekino, A.; Sugi, R.; Yokoyama, A. Chain-growth polycondensation for well-defined aramide. Synthesis of unprecedented block copolymer containing aramide with low polydispersity. J. Am. Chem. Soc. 2002, 124, 15158–15159.
- 54 Toth, J. M.; Wang, M.; Estes, B. T.; Scifert, J. L.; Seim, H. B.; Turner, A. S. Polyetheretherketone as a biomaterial for spinal applications. Biomaterials 2006, 27, 324–334.
- 55 Alqurashi, H.; Khurshid, Z.; Syed, A. U.; Habib, S. R.; Rokaya, D.; Zafar, M. S. Polyetherketoneketone (PEKK): An emerging biomaterial for oral implants and dental prostheses. J. Adv. Res. 2021, 28, 87–95.
- 56 Wang, F.; Hickner, M.; Kim, Y. S.; Zawodzinski, T. A.; McGrath, J. E. Direct polymerization of sulfonated poly (arylene ether sulfone) random (statistical) copolymers: candidates for new proton exchange membranes. J. Membr. Sci. 2002, 197, 231–242.
- 57 Kel'in, A. V.; Maioli, A. Recent advances in the chemistry of 1,3-diketones: Structural modifications and synthetic applications. Curr. Org. Chem. 2003, 7, 1855–1886.
- 58 Kel'in, A. V. Recent advances in the synthesis of 1,3-diketones. Curr. Org. Chem. 2003, 7, 1691–1711.
- 59 Lu, S. C.; Wen, F. Q.; Guan, X. D. Formal metal-free gamma-arylation of 1,3-dicarbonyl compounds via an isomerisation/1,4-addition/3,3- sigmatropic rearrangement sequence. Green Chem. 2021, 23, 8964–8968.
- 60 Hudson, B. E.; Hauser, C. R. The synthesis of ketones of the type RCOCHR2 from alpha, alpha-disubstituted beta-keto esters. An extension of the acetoacetic ester type of ketone synthesis. J. Am. Chem. Soc. 1941, 63, 3163–3164.
- 61 Peng, J. S.; Chen, T. H.; Chen, C. X.; Li, B. Palladium-Catalyzed intramolecular C-H Activation/C-C Bond Formation: A Straightforward Synthesis of Phenanthridines. J. Org. Chem. 2011, 76, 9507–9513.
- 62 Yao, Q.; Liao, Y. T.; Lin, L. L.; Lin, X. B.; Ji, J.; Liu, X. H.; Feng, X. M. Efficient Synthesis of Chiral Trisubstituted 1,2-Allenyl Ketones by Catalytic Asymmetric Conjugate Addition of Malonic Esters to Enynes. Angew. Chem. Int. Ed. 2016, 55, 1859–1863.
- 63 Lin, Q. Y.; Chu, L. L.; Qing, F. L. Direct Introduction of Ethoxycarbonyldifluoromethyl-Group to Heteroarenes with Ethyl Bromodifluoroacetate via Visible-Light Photocatalysis. Chin. J. Chem. 2013, 31, 885–891.




