Applications of Rare Earth Promoted Transition Metal Sulfides in Electrocatalysis†
Wei Shen
State Key Laboratory of Applied Organic Chemistry, Key Laboratory of Nonferrous Metal Chemistry and Resources Utilization of Gansu Province, College of Chemistry and Chemical Engineering, Lanzhou University, Lanzhou, Gansu, 730000 China
These authors contributed equally.
Search for more papers by this authorJiamin Zhu
State Key Laboratory of Applied Organic Chemistry, Key Laboratory of Nonferrous Metal Chemistry and Resources Utilization of Gansu Province, College of Chemistry and Chemical Engineering, Lanzhou University, Lanzhou, Gansu, 730000 China
These authors contributed equally.
Search for more papers by this authorYang Hu
State Key Laboratory of Applied Organic Chemistry, Key Laboratory of Nonferrous Metal Chemistry and Resources Utilization of Gansu Province, College of Chemistry and Chemical Engineering, Lanzhou University, Lanzhou, Gansu, 730000 China
These authors contributed equally.
Search for more papers by this authorJie Yin
State Key Laboratory of Applied Organic Chemistry, Key Laboratory of Nonferrous Metal Chemistry and Resources Utilization of Gansu Province, College of Chemistry and Chemical Engineering, Lanzhou University, Lanzhou, Gansu, 730000 China
Search for more papers by this authorCorresponding Author
Yao Zheng
School of Chemical Engineering and Advanced Materials, The University of Adelaide, Adelaide, South Australia, 5005 Australia
E-mail: [email protected], [email protected]Search for more papers by this authorCorresponding Author
Pinxian Xi
State Key Laboratory of Applied Organic Chemistry, Key Laboratory of Nonferrous Metal Chemistry and Resources Utilization of Gansu Province, College of Chemistry and Chemical Engineering, Lanzhou University, Lanzhou, Gansu, 730000 China
E-mail: [email protected], [email protected]Search for more papers by this authorWei Shen
State Key Laboratory of Applied Organic Chemistry, Key Laboratory of Nonferrous Metal Chemistry and Resources Utilization of Gansu Province, College of Chemistry and Chemical Engineering, Lanzhou University, Lanzhou, Gansu, 730000 China
These authors contributed equally.
Search for more papers by this authorJiamin Zhu
State Key Laboratory of Applied Organic Chemistry, Key Laboratory of Nonferrous Metal Chemistry and Resources Utilization of Gansu Province, College of Chemistry and Chemical Engineering, Lanzhou University, Lanzhou, Gansu, 730000 China
These authors contributed equally.
Search for more papers by this authorYang Hu
State Key Laboratory of Applied Organic Chemistry, Key Laboratory of Nonferrous Metal Chemistry and Resources Utilization of Gansu Province, College of Chemistry and Chemical Engineering, Lanzhou University, Lanzhou, Gansu, 730000 China
These authors contributed equally.
Search for more papers by this authorJie Yin
State Key Laboratory of Applied Organic Chemistry, Key Laboratory of Nonferrous Metal Chemistry and Resources Utilization of Gansu Province, College of Chemistry and Chemical Engineering, Lanzhou University, Lanzhou, Gansu, 730000 China
Search for more papers by this authorCorresponding Author
Yao Zheng
School of Chemical Engineering and Advanced Materials, The University of Adelaide, Adelaide, South Australia, 5005 Australia
E-mail: [email protected], [email protected]Search for more papers by this authorCorresponding Author
Pinxian Xi
State Key Laboratory of Applied Organic Chemistry, Key Laboratory of Nonferrous Metal Chemistry and Resources Utilization of Gansu Province, College of Chemistry and Chemical Engineering, Lanzhou University, Lanzhou, Gansu, 730000 China
E-mail: [email protected], [email protected]Search for more papers by this authorDedicated to the Special Issue of Energy Materials.
Comprehensive Summary
With the rapid consumption of fossil fuels and the resulting environmental problems, researchers are working to find sustainable alternative energy and energy storage and conversion methods. Transition metal sulfur compounds have attracted extensive attention due to their excellent electrical conductivity, low cost, adjustable components and good electrocatalytic performance. As an alternative to noble metal catalysts, they have emerged as a promising electrocatalyst. However, their low catalytic activity and poor stability limit their large-scale practical applications. Rare earth elements, known as industrial vitamins, are widely used in various fields due to their special redox properties, oxygen affinity and electronic structure. Therefore, the construction of rare earth promoted transition metal sulfides is of far-reaching significance for the development of catalysts. Here, we review the applications of various rare earth promoted transition metal sulfides in energy storage and conversion in recent years, which focuses on three ways in rare earth promoted transition metal sulfide, including doping, interfacial modification engineering and structural facilitation. As well, these materials are used in electrochemical reactions such as OER, HER, ORR, CO2RR, and so on, in order to explore the important role of rare earth in the field of electrocatalysis, the future challenges and opportunities.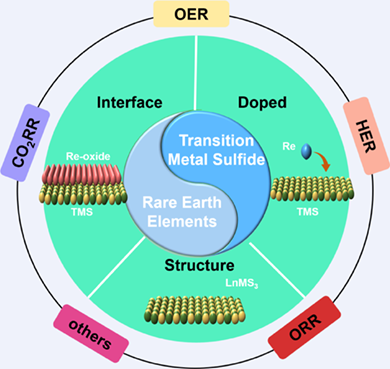
References
- 1 Li, Y.; Wang, Y.; Pattengale, B.; Yin, J.; An, L.; Cheng, F.; Li, Y.; Huang, J.; Xi, P. High-index faceted CuFeS2 nanosheets with enhanced behavior for boosting hydrogen evolution reaction. Nanoscale 2017, 9, 9230–9237.
- 2 Zhao, J.; Ren, X.; Sun, X.; Zhang, Y.; Wei, Q.; Liu, X.; Wu, D. In situ evolution of surface Co2CrO4 to CoOOH/CrOOH by electrochemical method: Toward boosting electrocatalytic water oxidation. Chin. J. Chem. 2021, 42, 1096–1101.
- 3 Wang, K.; Wang, X.; Li, Z.; Yang, B.; Ling, M.; Gao, X.; Lu, J.; Shi, Q.; Lei, L.; Wu, G.; Hou, Y. Designing 3d dual transition metal electrocatalysts for oxygen evolution reaction in alkaline electrolyte: Beyond oxides. Nano Energy 2020, 77, 105162.
- 4 Cheisson, T.; Schelter, E. J. Rare earth elements: Mendeleev's bane, modern marvels. Science 2019, 363, 489–493.
- 5 Swain, N.; Mishra, S. A review on the recovery and separation of rare earths and transition metals from secondary resources. J. Clean. Prod. 2019, 220, 884–898.
- 6 Xu, W.; Wang, G.; Liu, Q.; Sun, X.; Ma, X.; Cheng, Z.; Wu, J.; Shi, M.; Zhu, J.; Qi, Y. Doping vacancy synergy engineering: Ce-doped FeNiSx micro-succulent ameliorating electrocatalytic oxygen evolution performance. Electrochim. Acta 2022, 431, 141133.
- 7 Zhang, L.; Zhang, L.; Xu, G.; Zhang, C.; Li, X.; Sun, Z.; Jia, D. Low-temperature CO oxidation over CeO2 and CeO2@Co3O4 core–shell microspheres. New J. Chem. 2017, 41, 13418–13424.
- 8 Wu, L.-M.; Seo, D.-K. New Solid−Gas Metathetical Synthesis of Binary Metal Polysulfides and Sulfides at Intermediate Temperatures: Utilization of Boron Sulfides. J. Am. Chem. Soc. 2004, 126, 4676–4681.
- 9 Wang, H.-F.; Tang, C.; Wang, B.; Li, B.-Q.; Zhang, Q. Bifunctional Transition Metal Hydroxysulfides: Room-Temperature Sulfurization and Their Applications in Zn–Air Batteries. Adv. Mater. 2017, 29, 1702327.
- 10 He, R.; Huang, X.; Feng, L. Recent Progress in Transition-Metal Sulfide Catalyst Regulation for Improved Oxygen Evolution Reaction. Energy Fuels 2022, 36, 6675–6694.
- 11 He, W.; Wang, F.; Gao, Y.; Hao, Q.; Liu, C. One-step synthesis of amorphous transition metal sulfides as bifunctional electrocatalysts for the hydrogen evolution reaction and oxygen evolution reaction. Sustainable Energy Fuels 2022, 6, 3852–3857.
- 12 Li, M.; Zheng, K.; Zhang, J.; Li, X.; Xu, C. Design and construction of 2D/2D sheet-on-sheet transition metal sulfide/phosphide heterostructure for efficient oxygen evolution reaction. Appl. Surf. Sci. 2021, 565, 150510.
- 13 Peng, L.; Shah, S. S. A.; Wei, Z. Recent developments in metal phosphide and sulfide electrocatalysts for oxygen evolution reaction. Chin. J. Chem. 2018, 39, 1575–1593.
- 14 Weeks, M. E. The discovery of the elements. XVI. The rare earth elements. J. Chem. Educ. 1932, 9, 1751.
- 15 Li, H.; Wang, P.; Lin, G.; Huang, J. The role of rare earth elements in biodegradable metals: A review. Acta Biomater.2021, 129, 33–42.
- 16 Shiraz, H. G.; Crispin, X.; Berggren, M. Transition metal sulfides for electrochemical hydrogen evolution. Int. J. Hydrog. Energy 2021, 46, 24060–24077.
- 17 Ma, M.; Yao, Y.; Wu, Y.; Yu, Y. Progress and Prospects of Transition Metal Sulfides for Sodium Storage. Adv. Fiber Mater. 2020, 2, 314–337.
- 18 Lin, Z.; Carvalho, B. R.; Kahn, E.; Lv, R.; Rao, R.; Terrones, H.; Pimenta, M. A.; Terrones, M. Defect engineering of two-dimensional transition metal dichalcogenides. 2D Mater. 2016, 3, 022002.
- 19 Wu, Z.; Huang, L.; Liu, H.; Li, M.; Wang, H. Surface oxidation of transition metal sulfide and phosphide nanomaterials. Nano Res. 2021, 14, 2264–2267.
- 20 Guo, Y.; Park, T.; Yi, J. W.; Henzie, J.; Kim, J.; Wang, Z.; Jiang, B.; Bando, Y.; Sugahara, Y.; Tang, J.; Yamauchi, Y. Nanoarchitectonics for Transition-Metal-Sulfide-Based Electrocatalysts for Water Splitting. Adv. Mater. 2019, 31, 1807134.
- 21 Zhou, J.-E.; Chen, J.; Peng, Y.; Zheng, Y.; Zeb, A.; Lin, X. Metal-organic framework-derived transition metal sulfides and their composites for alkali-ion batteries: A review. Coord. Chem. Rev. 2022, 472, 214781.
- 22 Lu, Q.; Wu, H.; Zheng, X.; Cao, Y.; Li, J.; Wang, Y.; Wang, H.; Zhi, C.; Deng, Y.; Han, X.; Hu, W. Controllable Constructing Janus Homologous Heterostructures of Transition Metal Alloys/Sulfides for Efficient Oxygen Electrocatalysis. Adv. Energy Mater. 2022, 12, 2202215.
- 23 Wang, W.; Qi, J.; Zhai, L.; Ma, C.; Ke, C.; Zhai, W.; Wu, Z.; Bao, K.; Yao, Y.; Li, S.; Chen, B.; Repaka, D. V. M.; Zhang, X.; Ye, R.; Lai, Z.; Luo, G.; Chen, Y.; He, Q. Preparation of 2D Molybdenum Phosphide via Surface-Confined Atomic Substitution. Adv. Mater. 2022, 34, e2203220.
- 24 Taghinejad, H.; Taghinejad, M.; Eftekhar, A. A.; Li, Z.; West, M. P.; Javani, M. H.; Abdollahramezani, S.; Zhang, X.; Tian, M.; Johnson-Averette, T.; Ajayan, P. M.; Vogel, E. M.; Shi, S. F.; Cai, W.; Adibi, A. Synthetic Engineering of Morphology and Electronic Band Gap in Lateral Heterostructures of Monolayer Transition Metal Dichalcogenides. ACS Nano 2020, 14, 6323–6330.
- 25 Emadi, H.; Salavati-Niasari, M.; Sobhani, A. Synthesis of some transition metal (M25Mn27Co28Ni29Cu30Zn47Ag48Cd) sulfide nanostructures by hydrothermal method. Adv. Colloid Interface Sci. 2017, 246, 52–74.
- 26 Ng, S. F.; Lau, M. Y. L.; Ong, W. J. Lithium-Sulfur Battery Cathode Design: Tailoring Metal-Based Nanostructures for Robust Polysulfide Adsorption and Catalytic Conversion. Adv. Mater. 2021, 33, e2008654.
- 27 Qu, J.; Li, Y.; Li, F.; Li, T.; Wang, X.; Yin, Y.; Ma, L.; Schmidt, O. G.; Zhu, F. Direct Thermal Enhancement of Hydrogen Evolution Reaction of On-Chip Monolayer MoS2. ACS Nano 2022, 16, 2921–2927.
- 28 Tian, B.; Lei, J.; Wang, J. Polyoxometalate-Surfactant Hybrids Directed Assembly of Ni3S2 into Hollow Microsphere as Pt-Comparable Electrocatalyst for Hydrogen Evolution Reaction in Alkaline Medium. ACS Appl. Mater. Interfaces 2017, 9, 40162–40170.
- 29 Qin, J.-F.; Yang, M.; Chen, T.-S.; Dong, B.; Hou, S.; Ma, X.; Zhou, Y.-N.; Yang, X.-L.; Nan, J.; Chai, Y.-M. Ternary metal sulfides MoCoNiS derived from metal organic frameworks for efficient oxygen evolution. Int. J. Hydrog. Energy 2020, 45, 2745–2753.
- 30 Fu, G.; Lee, J.-M. Ternary metal sulfides for electrocatalytic energy conversion. J. Mater. Chem. A 2019, 7, 9386–9405.
- 31 Cheisson, T.; Schelter, E. J. Rare earth elements: Mendeleev's bane, modern marvels. Science 2019, 363, 489–493.
- 32 Turner, A.; Scott, J. W.; Green, L. A. Rare earth elements in plastics. Sci. Total Environ. 2021, 774, 145405.
- 33 Zhang, N.; Yan, H.; Li, L.; Wu, R.; Song, L.; Zhang, G.; Liang, W.; He, H. Use of rare earth elements in single-atom site catalysis: A critical review—Commemorating the 100th anniversary of the birth of Academician Guangxian Xu. J. Rare Earths 2021, 39, 233–242.
- 34 Wang, L.; Huang, X.; Yu, Y.; Zhao, L.; Wang, C.; Feng, Z.; Cui, D.; Long, Z. Towards cleaner production of rare earth elements from bastnaesite in China. J. Clean. Prod. 2017, 165, 231–242.
- 35 Zeng, Z.; Xu, Y.; Zhang, Z.; Gao, Z.; Luo, M.; Yin, Z.; Zhang, C.; Xu, J.; Huang, B.; Luo, F.; Du, Y.; Yan, C. Rare-earth-containing perovskite nanomaterials: design, synthesis, properties and applications. Chem. Soc. Rev. 2020, 49, 1109-1143.
- 36 Wang, X.; Zhang, Q.; Mao, S.; Cheng, W. A Theoretical Study on the Electronic Structure and Floatability of Rare Earth Elements (La, Ce, Nd and Y) Bearing Fluorapatite. Minerals 2019, 9, 500.
- 37 Zhang, S.; Saji, S. E.; Yin, Z.; Zhang, H.; Du, Y.; Yan, C. H. Rare-Earth Incorporated Alloy Catalysts: Synthesis, Properties, and Applications. Adv. Mater. 2021, 33, e2005988.
- 38 Hughes, I. D.; Dane, M.; Ernst, A.; Hergert, W.; Luders, M.; Poulter, J.; Staunton, J. B.; Svane, A.; Szotek, Z.; Temmerman, W. M. Lanthanide contraction and magnetism in the heavy rare earth elements. Nature 2007, 446, 650–653.
- 39 Sarkar, A.; Wang, Q.; Schiele, A.; Chellali, M. R.; Bhattacharya, S. S.; Wang, D.; Brezesinski, T.; Hahn, H.; Velasco, L.; Breitung, B. High-Entropy Oxides: Fundamental Aspects and Electrochemical Properties. Adv. Mater. 2019, 31, e1806236.
- 40 Yao, Y.; Farac, N. F.; Azimi, G. Supercritical Fluid Extraction of Rare Earth Elements from Nickel Metal Hydride Battery. ACS Sustainable Chem. Eng. 2017, 6, 1417–1426.
- 41 Huang, H.; Zhu, J. J. The electrochemical applications of rare earth- based nanomaterials. Analyst 2019, 144, 6789–6811.
- 42 Gao, W.; Wen, D.; Ho, J. C.; Qu, Y. Incorporation of rare earth elements with transition metal–based materials for electrocatalysis: a review for recent progress. Mater. Today Chem. 2019, 12, 266–281.
- 43 Yan, J.; Li, P.; Ji, Y.; Bian, H.; Li, Y.; Liu, S. Earth-abundant elements doping for robust and stable solar-driven water splitting by FeOOH. J. Mater. Chem. A 2017, 5, 21478–21485.
- 44 Shit, S.; Bolar, S.; Murmu, N. C.; Kuila, T. Minimal lanthanum-doping triggered enhancement in bifunctional water splitting activity of molybdenum oxide/sulfide heterostructure through structural evolution. Chem. Eng. J. 2022, 428, 131131.
- 45 Yousaf, M.; Akhtar, M. N.; Wang, B.; Noor, A. Preparations, optical, structural, conductive and magnetic evaluations of RE's (Pr, Y, Gd, Ho, Yb) doped spinel nanoferrites. Ceram. Int. 2020, 46, 4280-4288.
- 46 Ghoshal, D.; Kumar, R.; Koratkar, N. Controlled Re doping in MoS2 by chemical vapor deposition. Inorg. Chem. Commun. 2021, 123, 108329.
- 47 Kochat, V.; Apte, A.; Hachtel, J. A.; Kumazoe, H.; Krishnamoorthy, A.; Susarla, S.; Idrobo, J. C.; Shimojo, F.; Vashishta, P.; Kalia, R.; Nakano, A.; Tiwary, C. S.; Ajayan, P. M. Re Doping in 2D Transition Metal Dichalcogenides as a New Route to Tailor Structural Phases and Induced Magnetism. Adv. Mater. 2017, 29, 1703754.
- 48 Yang, H.; Peng, F.; Schier, D. E.; Markotic, S. A.; Zhao, X.; Hong, A. N.; Wang, Y.; Feng, P.; Bu, X. Selective Crystallization of Rare-Earth Ions into Cationic Metal-Organic Frameworks for Rare-Earth Separation. Angew. Chem. Int. Ed. 2021, 60, 11148–11152.
- 49 Song, L.; Liang, Z.; Sun, M.; Huang, B.; Du, Y. The interfacial effect induced by rare earth oxide in boosting the conversion of CO2 to formate. Energy Environ. Sci. 2022, 15, 3494–3502.
- 50 Quiñonero, J.; Lana–Villarreal, T.; Gómez, R. Improving the photoactivity of bismuth vanadate thin film photoanodes through doping and surface modification strategies. Appl. Catal. B 2016, 194, 141-149.
- 51 Li, X.; Wang, M.; Wang, R.; Shen, M.; Wu, P.; Fu, Z.; Zhu, M.; Zhang, L. A distinctive semiconductor-metalloid heterojunction: unique electronic structure and enhanced CO2 photoreduction activity. J. Colloid Interface Sci. 2022, 615, 821–830.
- 52 Wu, Q.; Gao, Q.; Sun, L.; Guo, H.; Tai, X.; Li, D.; Liu, L.; Ling, C.; Sun, X. Facilitating active species by decorating CeO2 on Ni3S2 nanosheets for efficient water oxidation electrocatalysis. Chin. J. Chem. 2021, 42, 482–489.
- 53 Xie, H.; Geng, Q.; Liu, X.; Mao, J. Interface engineering for enhancing electrocatalytic oxygen evolution reaction of CoS/CeO2 heterostructures. Front. Chem. Sci. Eng. 2021, 16, 376–383.
- 54 Rodney, J. D.; Deepapriya, S.; Robinson, M. C.; Das, S. J.; Perumal, S.; Sivakumar, P.; Jung, H.; Kim, B. C.; Raj, C. J. Cu1-xRExO (RE = La, Dy) decorated dendritic CuS nanoarrays for highly efficient splitting of seawater into hydrogen and oxygen fuels. Appl. Mater. Today 2021, 24, 101079.
- 55 Kozhakhmetov, A.; Schuler, B.; Tan, A. M. Z.; Cochrane, K. A.; Nasr, J. R.; El-Sherif, H.; Bansal, A.; Vera, A.; Bojan, V.; Redwing, J. M.; Bassim, N.; Das, S.; Hennig, R. G.; Weber-Bargioni, A.; Robinson, J. A. Scalable Substitutional Re-Doping and Its Impact on the Optical and Electronic Properties of Tungsten Diselenide. Adv. Mater. 2020, 32, e2005159.
- 56 Devendran, P.; Selvakumar, D.; Ramadoss, G.; Sivaramakrishnan, R.; Alagesan, T.; Jayavel, R.; Pandian, K. A novel visible light active rare earth doped CdS nanoparticles decorated reduced graphene oxide sheets for the degradation of cationic dye from wastewater. Chemosphere 2022, 287, 132091.
- 57 Li, Y.; Bunes, B. R.; Zang, L.; Zhao, J.; Li, Y.; Zhu, Y.; Wang, C. Atomic Scale Imaging of Nucleation and Growth Trajectories of an Interfacial Bismuth Nanodroplet. ACS Nano 2016, 10, 2386–2391.
- 58 Ng, J. W. D.; García-Melchor, M.; Bajdich, M.; Chakthranont, P.; Kirk, C.; Vojvodic, A.; Jaramillo, T. F. Gold-supported cerium-doped NiOx catalysts for water oxidation. Nat. Energy 2016, 1, 16053.
- 59 Xu, H.; Wang, B.; Shan, C.; Xi, P.; Liu, W.; Tang, Y. Ce-Doped NiFe-Layered Double Hydroxide Ultrathin Nanosheets/Nanocarbon Hierarchical Nanocomposite as an Efficient Oxygen Evolution Catalyst. ACS Appl. Mater. Interfaces 2018, 10, 6336-6345.
- 60 Zhang, W.; Zhang, L.; Guan, K.; Zhang, X.; Meng, J.; Wang, H.; Liu, X.; Meng, J. Effective promotion of oxygen reduction activity by rare earth doping in simple perovskite cathodes for intermediate-temperature solid oxide fuel cells. J. Power Sources 2020, 446, 227360.
- 61 Coduri, M.; Checchia, S.; Longhi, M.; Ceresoli, D.; Scavini, M. Rare Earth Doped Ceria: The Complex Connection Between Structure and Properties. Front. Chem. 2018, 6, 526.
- 62 Mehtab, A.; Ahmed, J.; Alshehri, S. M.; Mao, Y.; Ahmad, T. Rare earth doped metal oxide nanoparticles for photocatalysis: a perspective. Nanotechnology 2022, 33, 142001.
- 63 Gao, W.; Ma, F.; Wang, C.; Wen, D. Ce dopant significantly promotes the catalytic activity of Ni foam-supported Ni3S2 electrocatalyst for alkaline oxygen evolution reaction. J. Power Sources 2020, 450, 227654.
- 64 Yang, W.; Wang, X.; Song, S.; Zhang, H. Syntheses and Applications of Noble-Metal-free CeO2-Based Mixed-Oxide Nanocatalysts. Chem 2019, 5, 1743–1774.
- 65 Wang, J.; Xiao, X.; Liu, Y.; Pan, K.; Pang, H.; Wei, S. The application of CeO2-based materials in electrocatalysis. J. Mater. Chem. A 2019, 7, 17675–17702.
- 66 Li, J.; Xia, Z.; Xue, Q.; Zhang, M.; Zhang, S.; Xiao, H.; Ma, Y.; Qu, Y. Insights into the Interfacial Lewis Acid–Base Pairs in CeO2-Loaded CoS2 Electrocatalysts for Alkaline Hydrogen Evolution. Small 2021, 17, 2103018.
- 67 Wang, R.; Wei, Y.; An, L.; Yang, R.; Guo, L.; Weng, Z.; Da, P.; Chen, W.; Jin, J.; Li, J.; Xi, P. Construction and Application of Interfacial Inorganic Nanostructures. Chin. J. Chem. 2020, 38, 772–786.
- 68 Hu, M.; Cai, Z.; Liang, X.; Li, G.; Tan, H.; Yang, S.; Ren, H.; Wang, Z.; Sun, G.; Liu, S.; Hao, Z.; Zhou, K. Surface-Confined Synthesis of Ultrafine Pt-Rare Earth Nanoalloys on N-Functionalized Supports. Adv. Funct. 2022, 32, 2202675.
- 69 Feng, J. X.; Ye, S. H.; Xu, H.; Tong, Y. X.; Li, G. R. Design and Synthesis of FeOOH/CeO2 Heterolayered Nanotube Electrocatalysts for the Oxygen Evolution Reaction. Adv. Mater. 2016, 28, 4698–4703.
- 70 Long, X.; Lin, H.; Zhou, D.; An, Y.; Yang, S. Enhancing Full Water-Splitting Performance of Transition Metal Bifunctional Electrocatalysts in Alkaline Solutions by Tailoring CeO2–Transition Metal Oxides–Ni Nanointerfaces. ACS Energy Lett. 2018, 3, 290–296.
- 71 Xia, J.; Zhao, H.; Huang, B.; Xu, L.; Luo, M.; Wang, J.; Luo, F.; Du, Y.; Yan, C. H. Efficient Optimization of Electron/Oxygen Pathway by Constructing Ceria/Hydroxide Interface for Highly Active Oxygen Evolution Reaction. Adv. Funct. 2020, 30, 1908367.
- 72 Gao, W.; Xia, Z.; Cao, F.; Ho, J. C.; Jiang, Z.; Qu, Y. Comprehensive Understanding of the Spatial Configurations of CeO2 in NiO for the Electrocatalytic Oxygen Evolution Reaction: Embedded or Surface-Loaded. Adv. Funct. 2018, 28, 1706056.
- 73 Dai, T.; Zhang, X.; Sun, M.; Huang, B.; Zhang, N.; Da, P.; Yang, R.; He, Z.; Wang, W.; Xi, P.; Yan, C. H. Uncovering the Promotion of CeO2/ CoS1.97 Heterostructure with Specific Spatial Architectures on Oxygen Evolution Reaction. Adv. Mater. 2021, 33, e2102593.
- 74 Liao, J.; Xue, Z.; Sun, H.; Xue, F.; Zhao, Z.; Wang, X.; Dong, W.; Yang, D.; Nie, M. MoS2 supported on Er-MOF as efficient electrocatalysts for hydrogen evolution reaction. J. Alloys Compd. 2022, 898, 162991.
- 75 Murugesan, T.; Ramesh, S.; Gopalakrishnan, J.; Rao, C. N. R. Rare earth transition metal sulfides, LnMS3. J. Solid State Chem. 1981, 38, 165–172.
- 76 Yin, W.-J.; Weng, B.; Ge, J.; Sun, Q.; Li, Z.; Yan, Y. Oxide perovskites, double perovskites and derivatives for electrocatalysis, photocatalysis, and photovoltaics. Energy Environ. Sci. 2019, 12, 442–462.
- 77 Liu, Y.; Huang, H.; Xue, L.; Sun, J.; Wang, X.; Xiong, P.; Zhu, J. Recent advances in the heteroatom doping of perovskite oxides for efficient electrocatalytic reactions. Nanoscale 2021, 13, 19840–19856.
- 78 Shen, W.; Jin, J.; Hu, Y.; Hou, Y.; Yin, J.; Ma, Z.; Zhao, Y.-Q.; Xi, P. Surface chlorine doped perovskite-type cobaltate lanthanum for water oxidation. Chin. J. Chem. 2022, 43, 1485–1492.
- 79 Bao, B.; Liu, Y.; Sun, M.; Huang, B.; Hu, Y.; Da, P.; Ji, D.; Xi, P.; Yan, C. H. Boosting the Electrocatalytic Oxygen Evolution of Perovskite LaCo1-xFexO3 by the Construction of Yolk-Shell Nanostructures and Electronic Modulation. Small 2022, 18, e2201131.
- 80
Niu, Y.; Gong, S.; Liu, X.; Xu, C.; Xu, M.; Sun, S.-G.; Chen, Z. Engineering iron-group bimetallic nanotubes as efficient bifunctional oxygen electrocatalysts for flexible Zn–air batteries. eScience 2022, 2, 546–556.
10.1016/j.esci.2022.05.001 Google Scholar
- 81 Rao, P.; Wu, D.; Wang, T.-J.; Li, J.; Deng, P.; Chen, Q.; Shen, Y.; Chen, Y.; Tian, X. Single atomic cobalt electrocatalyst for efficient oxygen reduction reaction. eScience 2022, 2, 399–404.
- 82 Guo, X.; Wan, X.; Liu, Q.; Li, Y.; Li, W.; Shui, J. Phosphated IrMo bimetallic cluster for efficient hydrogen evolution reaction. eScience 2022, 2, 304–310.
- 83
Meng, F.; Dai, C.; Liu, Z.; Luo, S.; Ge, J.; Duan, Y.; Chen, G.; Wei, C.; Chen, R. R.; Wang, J.; Mandler, D.; Xu, Z. J. Methanol electro-oxidation to formate on iron-substituted lanthanum cobaltite perovskite oxides. eScience 2022, 2, 87–94.
10.1016/j.esci.2022.02.001 Google Scholar
- 84
Wang, W.; Wang, Z.; Hu, Y.; Liu, Y.; Chen, S. A potential-driven switch of activity promotion mode for the oxygen evolution reaction at Co3O4/NiOxHy interface. eScience 2022, 2, 438–444.
10.1016/j.esci.2022.04.004 Google Scholar
- 85 An, L.; Wei, C.; Lu, M.; Liu, H.; Chen, Y.; Scherer, G. G.; Fisher, A. C.; Xi, P.; Xu, Z. J.; Yan, C. H. Recent Development of Oxygen Evolution Electrocatalysts in Acidic Environment. Adv. Mater. 2021, 33, e2006328.
- 86 Wei, Y.; Zheng, Y.; Hu, Y.; Huang, B.; Sun, M.; Da, P.; Xi, P.; Yan, C. H. Controlling the Cation Exsolution of Perovskite to Customize Heterostructure Active Site for Oxygen Evolution Reaction. ACS Appl. Mater. Interfaces 2022, 14, 25638–25647.
- 87 Zhang, J.; Qian, J.; Ran, J.; Xi, P.; Yang, L.; Gao, D. Engineering Lower Coordination Atoms onto NiO/Co3O4 Heterointerfaces for Boosting Oxygen Evolution Reactions. ACS Catal. 2020, 10, 12376–12384.
- 88 An, L.; Feng, J.; Zhang, Y.; Wang, R.; Liu, H.; Wang, G.-C.; Cheng, F.; Xi, P. Epitaxial Heterogeneous Interfaces on N-NiMoO4/NiS2 Nanowires/ Nanosheets to Boost Hydrogen and Oxygen Production for Overall Water Splitting. Adv. Funct. 2019, 29, 1805298.
- 89 An, L.; Hu, Y.; Li, J.; Zhu, J.; Sun, M.; Huang, B.; Xi, P.; Yan, C. H. Tailoring Oxygen Reduction Reaction Pathway on Spinel Oxides via Surficial Geometrical-Site Occupation Modification Driven by the Oxygen Evolution Reaction. Adv. Mater. 2022, 34, e2202874.
- 90 Zhang, N.; Hu, Y.; An, L.; Li, Q.; Yin, J.; Li, J.; Yang, R.; Lu, M.; Zhang, S.; Xi, P.; Yan, C. H. Surface Activation and Ni-S Stabilization in NiO/NiS2 for Efficient Oxygen Evolution Reaction. Angew. Chem. Int. Ed. 2022, 61, e202207217.
- 91 Shen, W.; Yin, J.; Jin, J.; Hu, Y.; Hou, Y.; Xiao, J.; Zhao, Y.-Q.; Xi, P. Progress in In Situ Research on Dynamic Surface Reconstruction of Electrocatalysts for Oxygen Evolution Reaction. Adv. Energy Mater. 2022, 3, 2200036.
- 92 Yin, J.; Jin, J.; Lu, M.; Huang, B.; Zhang, H.; Peng, Y.; Xi, P.; Yan, C.-H. Iridium Single Atoms Coupling with Oxygen Vacancies Boosts Oxygen Evolution Reaction in Acid Media. J. Am. Chem. Soc. 2020, 142, 18378–18386.
- 93 Suntivich, J.; May, K. J.; Gasteiger, H. A.; Goodenough, J. B.; Shao-Horn, Y. A Perovskite Oxide Optimized for Oxygen Evolution Catalysis from Molecular Orbital Principles. Science 2011, 334, 1383–1385.
- 94 Xu, L.; Zhou, W.; Chao, S.; Liang, Y.; Zhao, X.; Liu, C.; Xu, J. Advanced Oxygen-Vacancy Ce-Doped MoO3 Ultrathin Nanoflakes Anode Materials Used as Asymmetric Supercapacitors with Ultrahigh Energy Density. Adv. Energy Mater.2022, 12, 2200101.
- 95 Song, J.; Wei, C.; Huang, Z. F.; Liu, C.; Zeng, L.; Wang, X.; Xu, Z. J. A review on fundamentals for designing oxygen evolution electrocatalysts. Chem. Soc. Rev. 2020, 49, 2196–2214.
- 96 Santhosh, G.; Nayaka, G. P.; Bhatt, A. S. Ultrahigh capacitance of NiCo2O4/CeO2 mixed metal oxide material for supercapacitor applications. J. Alloys Compd. 2022, 899, 163312.
- 97 Xu, H.; Cao, J.; Shan, C.; Wang, B.; Xi, P.; Liu, W.; Tang, Y. MOF-Derived Hollow CoS Decorated with CeOx Nanoparticles for Boosting Oxygen Evolution Reaction Electrocatalysis. Angew Chem. Int. Ed. 2018, 57, 8654–8658.
- 98 Zheng, Y. R.; Gao, M. R.; Gao, Q.; Li, H. H.; Xu, J.; Wu, Z. Y.; Yu, S. H. An efficient CeO2/CoSe2 Nanobelt composite for electrochemical water oxidation. Small 2015, 11, 182–188.
- 99 Zhu, W.; Huang, Z.; Zhao, M.; Huang, R.; Wang, Z.; Liang, H. Hydrogen production by electrocatalysis using the reaction of acidic oxygen evolution: a review. Environ. Chem. Lett. 2022, 3429–3452
- 100 An, L.; Zhang, Y.; Wang, R.; Liu, H.; Gao, D.; Zhao, Y.-Q.; Cheng, F.; Xi, P. Activation of defective nickel molybdate nanowires for enhanced alkaline electrochemical hydrogen evolution. Nanoscale 2018, 10, 16539–16546.
- 101 Yin, J.; Jin, J.; Zhang, H.; Lu, M.; Peng, Y.; Huang, B.; Xi, P.; Yan, C.-H. Atomic Arrangement in Metal-Doped NiS2 Boosts the Hydrogen Evolution Reaction in Alkaline Media. Angew Chem. Int. Ed. 2019, 58, 18676–18682.
- 102 Ge, M.; Zhang, X.; Xia, S.; Luo, W.; Jin, Y.; Chen, Q.; Nie, H.; Yang, Z. Uniform Formation of Amorphous Cobalt Phosphate on Carbon Nanotubes for Hydrogen Evolution Reaction. Chin. J. Chem. 2021, 39, 2113–2118.
- 103 Huang, C.; Yu, L.; Zhang, W.; Xiao, Q.; Zhou, J.; Zhang, Y.; An, P.; Zhang, J.; Yu, Y. N-doped Ni-Mo based sulfides for high-efficiency and stable hydrogen evolution reaction. Appl. Catal. B 2020, 276, 119137.
- 104 Wang, X.; Zheng, Y.; Sheng, W.; Xu, Z. J.; Jaroniec, M.; Qiao, S.-Z. Strategies for design of electrocatalysts for hydrogen evolution under alkaline conditions. Mater.Today 2020, 36, 125–138.
- 105 Tang, T.; Wang, Z.; Guan, J. A review of defect engineering in two-dimensional materials for electrocatalytic hydrogen evolution reaction. Chin. J. Chem. 2022, 43, 636–678.
- 106 Ding, X.; Yang, T.; Wei, W.; Wang, Y.; Xu, K.; Zhu, Z.; Zhao, H.; Yu, T.; Zhang, D. An in situ grown lanthanum sulfide/molybdenum sulfide hybrid catalyst for electrochemical hydrogen evolution. Catal. Sci. Technol. 2020, 10, 3247–3254.
- 107 Hu, J.; Liu, W.; Xin, C.; Guo, J.; Cheng, X.; Wei, J.; Hao, C.; Zhang, G.; Shi, Y. Carbon-based single atom catalysts for tailoring the ORR pathway: a concise review. J. Mater. Chem. A 2021, 9, 24803–24829.
- 108 Pang, R.; Xia, H.; Li, J.; Guo, S.; Wang, E. Recent Developments of Atomically Dispersed Metal Electrocatalysts for Oxygen Reduction Reaction. Chin. J. Chem. 2022, 41, 581–598.
- 109 Zhong, W.-X.; Zhao, X.-R.; Qin, J.-Y.; Yang, J. An Active Hybrid Electrocatalyst with Synergized Pyridinic Nitrogen-Cobalt and Oxygen Vacancies for Bifunctional Oxygen Reduction and Evolution. Chin. J. Chem. 2021, 39, 655–660.
- 110
Yang, G.; Liang, X.; Zheng, S.; Chen, H.; Zhang, W.; Li, S.; Pan, F. Li-rich channels as the material gene for facile lithium diffusion in halide solid electrolytes. eScience 2022, 2, 79–86.
10.1016/j.esci.2022.01.001 Google Scholar
- 111 Zhang, J.; Zhang, J.; He, F.; Chen, Y.; Zhu, J.; Wang, D.; Mu, S.; Yang, H. Y. Defect and Doping Co-Engineered Non-Metal Nanocarbon ORR Electrocatalyst. Nanomicro Lett.2021, 13, 65.
- 112 Cruz-Martínez, H.; Rojas-Chávez, H.; Matadamas-Ortiz, P. T.; Ortiz-Herrera, J. C.; López-Chávez, E.; Solorza-Feria, O.; Medina, D. I. Current progress of Pt-based ORR electrocatalysts for PEMFCs: An integrated view combining theory and experiment. Mater. Today Phys. 2021, 19, 100406.
- 113 Yang, L.; Shui, J.; Du, L.; Shao, Y.; Liu, J.; Dai, L.; Hu, Z. Carbon-Based Metal-Free ORR Electrocatalysts for Fuel Cells: Past, Present, and Future. Adv. Mater. 2019, 31, e1804799.
- 114 Li, J. C.; Qin, X.; Xiao, F.; Liang, C.; Xu, M.; Meng, Y.; Sarnello, E.; Fang, L.; Li, T.; Ding, S.; Lyu, Z.; Zhu, S.; Pan, X.; Hou, P. X.; Liu, C.; Lin, Y.; Shao, M. Highly Dispersive Cerium Atoms on Carbon Nanowires as Oxygen Reduction Reaction Electrocatalysts for Zn-Air Batteries. Nano Lett. 2021, 21, 4508–4515.
- 115 Li, H.; Wen, Y.; Jiang, M.; Yao, Y.; Zhou, H.; Huang, Z.; Li, J.; Jiao, S.; Kuang, Y.; Luo, S. Understanding of Neighboring Fe-N4-C and Co-N4-C Dual Active Centers for Oxygen Reduction Reaction. Adv. Funct. 2021, 31, 2011289.
- 116 Gao, Y.; Kong, D.; Liang, J.; Han, D.; Wang, B.; Yang, Q.-H.; Zhi, L. Inside-out dual-doping effects on tubular catalysts: Structural and chemical variation for advanced oxygen reduction performance. Nano Res. 2021, 15, 361–367.
- 117 Li, X.; You, S.; Du, J.; Dai, Y.; Chen, H.; Cai, Z.; Ren, N.; Zou, J. ZIF-67-derived Co3O4@carbon protected by oxygen-buffering CeO2 as an efficient catalyst for boosting oxygen reduction/evolution reactions. J. Mater. Chem. A 2019, 7, 25853–25864.
- 118 Kirsanova, M. A.; Okatenko, V. D.; Aksyonov, D. A.; Forslund, R. P.; Mefford, J. T.; Stevenson, K. J.; Abakumov, Artem M. Bifunctional OER/ORR catalytic activity in the tetrahedral YBaCo4O7.3 oxide. J. Mater. Chem. A 2019, 7, 330–341.
- 119 Meng, G.; Chang, Z.; Cui, X.; Tian, H.; Ma, Z.; Peng, L.; Chen, Y.; Chen, C.; Shi, J. SnO2/CeO2 nanoparticle-decorated mesoporous ZSM-5 as bifunctional electrocatalyst for HOR and ORR. Chem. Eng. J. 2021, 417, 127913.
- 120 Retuerto, M.; Calle-Vallejo, F.; Pascual, L.; Lumbeeck, G.; Fernandez-Diaz, M. T.; Croft, M.; Gopalakrishnan, J.; Peña, M. A.; Hadermann, J.; Greenblatt, M.; Rojas, S. La1.5Sr0.5NiMn0.5Ru0.5O6 Double Perovskite with Enhanced ORR/OER Bifunctional Catalytic Activity. ACS Appl. Mater. Interfaces 2019, 11, 21454–21464.
- 121 Huang, B.; Peng, L.; Yang, F.; Liu, Y.; Xie, Z. Improving ORR activity of carbon nanotubes by hydrothermal carbon deposition method. J. Energy Chem. 2017, 26, 712–718.
- 122 Ding, J.; Ji, S.; Wang, H.; Pollet, B. G.; Wang, R. Mesoporous CoS/N-doped Carbon as HER and ORR Bifunctional Electrocatalyst for Water Electrolyzers and Zinc-Air Batteries. ChemCatChem 2019, 11, 1026–1032.
- 123 Shi, L.; Zhang, Y.; Han, X.; Niu, D.; Sun, J.; Yang, J. Y.; Hu, S.; Zhang, X. SDS-modified Nanoporous Silver as an Efficient Electrocatalyst for Selectively Converting CO2 to CO in Aqueous Solution. Chin. J. Chem. 2019, 37, 337–341.
- 124 Banerjee, S.; Gerke, C. S.; Thoi, V. S. Guiding CO2RR Selectivity by Compositional Tuning in the Electrochemical Double Layer. Acc. Chem. Res. 2022, 55, 504–515.
- 125 Ferri, M.; Delafontaine, L.; Guo, S.; Asset, T.; Cristiani, P.; Campisi, S.; Gervasini, A.; Atanassov, P. Steering Cu-Based CO2RR Electrocatalysts’ Selectivity: Effect of Hydroxyapatite Acid/Base Moieties in Promoting Formate Production. ACS Energy Lett. 2022, 7, 2304–2310.
- 126 Lyu, F.; Hua, W.; Wu, H.; Sun, H.; Deng, Z.; Peng, Y. Structural and interfacial engineering of well-defined metal-organic ensembles for electrocatalytic carbon dioxide reduction. Chin. J. Chem. 2022, 43, 1417–1432.
- 127 Wei, X.; Wei, S.; Cao, S.; Hu, Y.; Zhou, S.; Liu, S.; Wang, Z.; Lu, X. Cu acting as Fe activity promoter in dual-atom Cu/Fe-NC catalyst in CO2RR to C1 products. Appl. Surf. Sci. 2021, 564, 150423.
- 128 Woldu, A. R.; Huang, Z.; Zhao, P.; Hu, L.; Astruc, D. Electrochemical CO2 reduction (CO2RR) to multi-carbon products over copper-based catalysts. Coord. Chem. Rev. 2022, 454, 214340.
- 129 Hu, F.; Liao, L.; Chi, B.; Wang, H. Rare earth praseodymium-based single atom catalyst for high performance CO2 reduction reaction. Chem. Eng. J. 2022, 436, 135271.
- 130 Daiyan, R.; Tan, X.; Chen, R.; Saputera, W. H.; Tahini, H. A.; Lovell, E.; Ng, Y. H.; Smith, S. C.; Dai, L.; Lu, X.; Amal, R. Electroreduction of CO2 to CO on a Mesoporous Carbon Catalyst with Progressively Removed Nitrogen Moieties. ACS Energy Lett. 2018, 3, 2292–2298.
- 131 Peng, C.; Luo, G.; Zhang, J.; Chen, M.; Wang, Z.; Sham, T. K.; Zhang, L.; Li, Y.; Zheng, G. Double sulfur vacancies by lithium tuning enhance CO2 electroreduction to n-propanol. Nat. Commun. 2021, 12, 1580.
- 132 Yan, X.; Chen, C.; Wu, Y.; Liu, S.; Chen, Y.; Feng, R.; Zhang, J.; Han, B. Efficient electroreduction of CO2 to C2+ products on CeO2 modified CuO. Chem. Sci. 2021, 12, 6638–6645.
- 133 Zhou, X.; Shan, J.; Chen, L.; Xia, B. Y.; Ling, T.; Duan, J.; Jiao, Y.; Zheng, Y.; Qiao, S. Z. Stabilizing Cu2+ Ions by Solid Solutions to Promote CO2 Electroreduction to Methane. J. Am. Chem. Soc. 2022, 144, 2079–2084.
- 134 Yao, Y.; Wang, H.; Yuan, X.-z.; Li, H.; Shao, M. Electrochemical Nitrogen Reduction Reaction on Ruthenium. ACS Energy Lett. 2019, 4, 1336–1341.
- 135 Tang, C.; Qiao, S.-Z. True or False in Electrochemical Nitrogen Reduction. Joule 2019, 3, 1573–1575.
- 136 Liu, Y.; Deng, P.; Wu, R.; Zhang, X.; Sun, C.; Li, H. Oxygen vacancies for promoting the electrochemical nitrogen reduction reaction. J. Mater. Chem. A 2021, 9, 6694–6709.
- 137
Wang, T.; Cao, X.; Jiao, L. Ni2P/NiMoP heterostructure as a bifunctional electrocatalyst for energy-saving hydrogen production. eScience 2021, 1, 69–74.
10.1016/j.esci.2021.09.002 Google Scholar
- 138 Wen, W.; Du, X.; Zhang, X. Controlled synthesis of M doped N-Ni3S2 (M = Cu, Fe, Co and Ce) on Ni foam as efficient electrocatalyst for urea oxidation reaction and oxygen evolution reaction. J. Alloys Compd. 2022, 918, 165739.




