Harpagide Inhibits Microglial Activation and Protects Dopaminergic Neurons as Revealed by Nanoelectrode Amperometry†
Fu-Li Zhang
College of Chemistry and Molecular Sciences, Wuhan University, Wuhan, Hubei, 430072 China
‡ These authors contributed equally to this work.
† Dedicate to the Special Issue of In Situ Target Biomolecule Analysis in Confined Nanospace
Search for more papers by this authorYun Tang
College of Chemistry and Molecular Sciences, Wuhan University, Wuhan, Hubei, 430072 China
‡ These authors contributed equally to this work.
† Dedicate to the Special Issue of In Situ Target Biomolecule Analysis in Confined Nanospace
Search for more papers by this authorHong Jiang
College of Chemistry and Molecular Sciences, Wuhan University, Wuhan, Hubei, 430072 China
Search for more papers by this authorXiao-Ke Yang
College of Chemistry and Molecular Sciences, Wuhan University, Wuhan, Hubei, 430072 China
Search for more papers by this authorCorresponding Author
Wei-Hua Huang
College of Chemistry and Molecular Sciences, Wuhan University, Wuhan, Hubei, 430072 China
E-mail: [email protected]Search for more papers by this authorFu-Li Zhang
College of Chemistry and Molecular Sciences, Wuhan University, Wuhan, Hubei, 430072 China
‡ These authors contributed equally to this work.
† Dedicate to the Special Issue of In Situ Target Biomolecule Analysis in Confined Nanospace
Search for more papers by this authorYun Tang
College of Chemistry and Molecular Sciences, Wuhan University, Wuhan, Hubei, 430072 China
‡ These authors contributed equally to this work.
† Dedicate to the Special Issue of In Situ Target Biomolecule Analysis in Confined Nanospace
Search for more papers by this authorHong Jiang
College of Chemistry and Molecular Sciences, Wuhan University, Wuhan, Hubei, 430072 China
Search for more papers by this authorXiao-Ke Yang
College of Chemistry and Molecular Sciences, Wuhan University, Wuhan, Hubei, 430072 China
Search for more papers by this authorCorresponding Author
Wei-Hua Huang
College of Chemistry and Molecular Sciences, Wuhan University, Wuhan, Hubei, 430072 China
E-mail: [email protected]Search for more papers by this authorMain observation and conclusion
Parkinson's disease (PD) is one of the most common neurogenerative diseases (NDDs), characterized as less neurotransmitter release and loss of dopaminergic (DAergic) neurons with microglial inflammatory response as a key player. Natural product harpagide with anti-inflammatory function is a potential therapeutic drug of PD, but its role towards microglial activation and inflammation-mediated neuronal injury remained unsure. In this work, taking advantage of nanoelectrode amperometry with high temporal-spatial resolution, we used nanowire electrodes (NWEs) to monitor intracellular reactive oxygen species (ROS) level and carbon fiber nanoelectrodes (CFNEs) to detect synaptic dopamine exocytosis, to explore the effect of harpagide in modulating microglial inflammatory reaction and protecting DAergic neurons in neuron-microglia co-culture system. The results indicate that harpagide inhibits microglia from activation induced by LPS/IFN-γ and generation of ROS, therefore reduces inflammation-mediated neural injury and maintains dopamine exocytosis function. These conclusions establish that harpagide possesses promising avenues for preventive or therapeutic interventions against PD and other NDDs.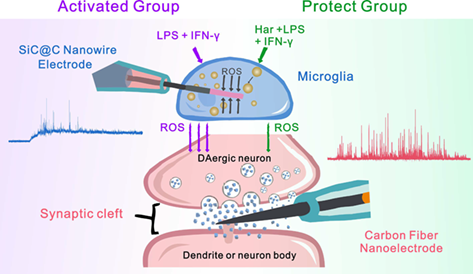
Supporting Information
| Filename | Description |
|---|---|
| cjoc202100178-sup-0001-Supinfo.pdfPDF document, 812 KB |
Appendix S1: Supporting Information |
Please note: The publisher is not responsible for the content or functionality of any supporting information supplied by the authors. Any queries (other than missing content) should be directed to the corresponding author for the article.
References
- 1 Lau, L. M. L. D.; Breteler, M. M. B. Epidemiology of Parkinson's Disease. Lancet Neurol. 2006, 5, 525–535.
- 2 Goedert, M.; Spillantini, M. G. A Century of Alzheimer's Disease. Science 2006, 314, 777–781.
- 3 Davie, C. A. A Review of Parkinson's Disease. Br. Med. Bull. 2008, 86, 109–127.
- 4 Carli, M.; Evenden, J. L.; Robbins, T. W. Depletion of Unilateral Striatal Dopamine Impairs Initiation of Contralateral Actions and Not Sensory Attention. Nature 1985, 313, 679–682.
- 5 Innis, R. B.; Seibyl, J. P.; Scanley, B. E.; Laruelle, M.; Abi-Dargham, A.; Wallace, E.; Baldwin, R. M.; Zea-Ponce, Y.; Zoghbi, S.; Wang, S.; Gao, Y.; Neumeyer, J. L.; Charney, D. S.; Hoffer, P. B.; Marek, K. L. Single Photon Emission Computed Tomographic Imaging Demonstrates Loss of Striatal Dopamine Transporters in Parkinson Disease. Proc. Natl. Acad. Sci. U. S. A. 1993, 90, 11965–11969.
- 6 Coyle, J. T.; Puttfarcken, P. Oxidative Stress, Glutamate, and Neurodegenerative Disorders. Science 1993, 262, 689–695.
- 7 Barnham, K. J.; Masters, C. L.; Bush, A. I. Neurodegenerative Diseases and Oxidative Stress. Nat. Rev. Drug Discovery 2004, 3, 205–214.
- 8 Sarkar, S.; Malovic, E.; Harishchandra, D. S.; Ghaisas, S.; Panicker, N.; Charli, A.; Palanisamy, B. N.; Rokad, D.; Jin, H.; Anantharam, V.; Kanthasamy, A.; Kanthasamy, A. G. Mitochondrial Impairment in Microglia Amplifies NLRP3 Inflammasome Proinflammatory Signaling in Cell Culture and Animal Models of Parkinson's Disease. npj Parkinson's Dis. 2017, 3, 30.
- 9 Che, Y.; Hou, L.; Sun, F.; Zhang, C.; Liu, X.; Piao, F.; Zhang, D.; Li, H.; Wang, Q. Taurine Protects Dopaminergic Neurons in a Mouse Parkinson's Disease Model Through Inhibition of Microglial M1 Polarization. Cell Death Dis. 2018, 9, 435.
- 10 Szalay, G.; Martinecz, B.; Lénárt, N.; Környei, Z.; Orsolits, B.; Judák, L.; Császár, E.; Fekete, R.; West, B. L.; Katona, G.; Rózsa, B.; Dénes, A. Microglia Protect Against Brain Injury and Their Selective Elimination Dysregulates Neuronal Network Activity after Stroke. Nat. Commun. 2016, 7, 11499.
- 11 Butovsky, O.; Weiner, H. L. Microglial Signatures and Their Role in Health and Disease. Nat. Rev. Neurosci. 2018, 19, 622–635.
- 12 McGeer, P. L.; Itagaki, S.; Boyes, B. E.; McGeer, E. G. Reactive Microglia Are Positive for HLA-DR in the Substantia Nigra of Parkinson's and Alzheimer's Disease Brains. Neurology 1988, 38, 1285–1291.
- 13 Gao, H. M.; Jiang, J.; Wilson, B.; Zhang, W.; Hong, J. S.; Liu, B. Microglial Activation-Mediated Delayed and Progressive Degeneration of Rat Nigral Dopaminergic Neurons: Relevance to Parkinson's Disease. J. Neurochem. 2002, 81, 1285–1297.
- 14 Block, M. L.; Hong, J. S. Microglia and Inflammation-Mediated Neurodegeneration: Multiple Triggers with a Common Mechanism. Prog. Neurobiol. 2005, 76, 77–98.
- 15 Lynch, M. A. The Multifaceted Profile of Activated Microglia. Mol. Neurobiol. 2009, 40, 139–156.
- 16 Hickman, S.; Izzy, S.; Sen, P.; Morsett, L.; El Khoury, J. Microglia in Neurodegeneration. Nat. Neurosci. 2018, 21, 1359–1369.
- 17 Block, M. L.; Zecca, L.; Hong, J. S. Microglia-Mediated Neurotoxicity: Uncovering the Molecular Mechanisms. Nat. Rev. Neurosci. 2007, 8, 57–69.
- 18 Fiebich, B. L.; Heinrich, M.; Hiller, K. O.; Kammerer, N. Inhibition of TNF-α Synthesis in LPS-Stimulated Primary Human Monocytes by Harpagophytum Extract SteiHap 69. Phytomedicine 2001, 8, 28–30.
- 19 Gyurkovska, V.; Alipieva, K.; Maciuk, A.; Dimitrova, P.; Ivanovska, N.; Haas, C.; Bley, T.; Georgiev, M. Anti-Inflammatory Activity of Devil's Claw In Vitro Systems and Their Active Constituents. Food Chem. 2011, 125, 171–178.
- 20 Zhang, L.; Feng, L.; Jia, Q.; Xu, J.; Wang, R.; Wang, Z.; Wu, Y.; Li, Y. Effects of β-glucosidase Hydrolyzed Products of Harpagide and Harpagoside on Cyclooxygenase-2 (COX-2) In Vitro. Bioorg. Med. Chem. 2011, 19, 4882–4886.
- 21 Schopohl, P.; Grüneberg, P.; Melzig, M. F. The Influence of Harpagoside and Harpagide on TNFα-Secretion and Cell Adhesion Molecule mRNA-Expression in IFN-γ/LPS-stimulated THP-1 Cells. Fitoterapia 2016, 110, 157–165.
- 22 Tang, Y.; Qiu, Q. F.; Zhang, F. L.; Xie, M.; Huang, W. H. Quantifying Orientational Regeneration of Injured Neurons by Natural Product Concentration Gradients in a 3D Microfluidic Device. Lab Chip 2018, 18, 971–978.
- 23 Tang, Y.; Yang, X. K.; Zhang, X. W.; Wu, W. T.; Zhang, F. L.; Jiang, H.; Liu, Y. L.; Amatore, C.; Huang, W. H. Harpagide, a Natural Product, Promotes Synaptic Vesicle Release as Measured by Nanoelectrode Amperometry. Chem. Sci. 2020, 11, 778–785.
- 24 Wightman, R. M. Probing Cellular Chemistry in Biological Systems with Microelectrodes. Science 2006, 311, 1570–1574.
- 25 Phan, N. T. N.; Li, X.; Ewing, A. G. Measuring Synaptic Vesicles Using Cellular Electrochemistry and Nanoscale Molecular Imaging. Nat. Rev. Chem. 2017, 1, 0048.
- 26 Zhou, J.; Jiang, D.; Chen, H. Y. Nanoelectrochemical Architectures for High-Spatial-Resolution Single Cell Analysis. Sci. China Chem. 2017, 60, 1277–1284.
- 27 Li, J.; Peng, Z.; Wang, E. Tackling Grand Challenges of the 21st Century with Electroanalytical Chemistry. J. Am. Chem. Soc. 2018, 140, 10629–10638.
- 28 Ying, Y. L.; Hu, Y. H.; Gao, R.; Yu, R. J.; Zhen, G.; Lee, L. P.; Long, Y. T. Asymmetric Nanopore Electrode Based Amplification for Electron Transfer Imaging in Live Cells. J. Am. Chem. Soc. 2018, 140, 5385–5392.
- 29 Hu, K.; Liu, Y. L.; Oleinick, A.; Mirkin, M. V.; Huang, W. H.; Amatore, C. Nanoelectrodes for Intracellular Measurements of Reactive Oxygen and Nitrogen Species in Single Living Cells. Curr. Opin. Electrochem. 2020, 22, 44–50.
- 30 Zhang, X. W.; Qiu, Q. F.; Jiang, H.; Zhang, F. L.; Liu, Y. L.; Amatore, C.; Huang, W. H. Real-Time Intracellular Measurements of ROS and RNS in Living Cells with Single Core-Shell Nanowire Electrodes. Angew. Chem. Int. Ed. 2017, 56, 12997–13000.
- 31 Zhang, X. W.; Oleinick, A.; Jiang, H.; Liao, Q. L.; Qiu, Q. F.; Svir, I.; Liu, Y. L.; Amatore, C.; Huang, W. H. Electrochemical Monitoring of ROS/RNS Homeostasis within Individual Phagolysosomes inside Single Macrophages. Angew. Chem. Int. Ed. 2019, 58, 7753–7756.
- 32 Jiang, H.; Zhang, X. W.; Liao, Q. L.; Wu, W. T.; Liu, Y. L.; Huang, W. H. Electrochemical Monitoring of Paclitaxel-Induced ROS Release from Mitochondria inside Single Cells. Small 2019, 15, 1901787.
- 33 Li, X.; Majdi, S.; Dunevall, J.; Fathali, H.; Ewing, A. G. Quantitative Measurement of Transmitters in Individual Vesicles in the Cytoplasm of Single Cells with Nanotip Electrodes. Angew. Chem. Int. Ed. 2015, 54, 11978–11982.
- 34 Li, X.; Ren, L.; Dunevall, J.; Ye, D.; White, H. S.; Edwards, M. A.; Ewing, A. G. Nanopore Opening at Flat and Nanotip Conical Electrodes during Vesicle Impact Electrochemical Cytometry. ACS Nano 2018, 12, 3010–3019.
- 35 Whitton, P. S. Inflammation as a Causative Factor in the Aetiology of Parkinson's Disease. Br. J. Pharmacol. 2007, 150, 963–976.
- 36 Li, Y. T.; Zhang, S. H.; Wang, L.; Xiao, R. R.; Liu, W.; Zhang, X. W.; Zhou, Z.; Amatore, C.; Huang, W. H. Nanoelectrode for Amperometric Monitoring of Individual Vesicular Exocytosis inside Single Synapses. Angew. Chem. Int. Ed. 2014, 53, 12456–12460.
- 37 Li, Y. T.; Zhang, S. H.; Wang, X. Y.; Zhang, X. W.; Oleinick, A. I.; Svir, I.; Amatore, C.; Huang, W. H. Real-time Monitoring of Discrete Synaptic Release Events and Excitatory Potentials within Self-Reconstructed Neuromuscular Junctions. Angew. Chem. Int. Ed. 2015, 54, 9313–9318.
- 38 Luk, K. C.; Song, C.; O'Brien, P.; Stieber, A.; Branch, J. R.; Brunden, K. R.; Trojanowski, J. Q.; Lee, V. M. Y. Exogenous α-Synuclein Fibrils Seed the Formation of Lewy Body-Like Intracellular Inclusions in Cultured Cells. Proc. Natl. Acad. Sci. U. S. A. 2009, 106, 20051–20056.
- 39 Nemani, V. M.; Lu, W.; Berge, V.; Nakamura, K.; Onoa, B.; Lee, M. K.; Chaudhry, F. A.; Nicoll, R. A.; Edwards, R. H. Increased Expression of Alpha-Synuclein Reduces Neurotransmitter Release by Inhibiting Synaptic Vesicle Reclustering after Endocytosis. Neuron 2010, 65, 66–79.
- 40 Vekrellis, K.; Xilouri, M.; Emmanouilidou, E.; Rideout, H. J.; Stefanis, L. Pathological Roles of Alpha-Synuclein in Neurological Disorders. Lancet Neurol. 2011, 10, 1015–1025.
- 41 Selkoe, D. J. Showing Transmitters the Door: Synucleins Accelerate Vesicle Release. Nat. Neurosci. 2017, 20, 629–631.
- 42 Söllner, T.; Bennett, M. K.; Whiteheart, S. W.; Scheller, R. H.; Rothman, J. E. A Protein Assembly-Disassembly Pathway In Vitro that May Correspond to Sequential Steps of Synaptic Vesicle Docking, Activation, and Fusion. Cell 1993, 75, 409–418.
- 43 Zhong, C.; Ying, Y.; Zhang, W.; Long, Y. Design and Simulation of Four-channel Electrochemical Sensors for Nanopore Analysis. Sci. China Chem. 2021, 51, 395–400.
- 44 Xu, C. Y.; Yu, R. J.; Ni, X.; Xu, S. W.; Fu, X. X.; Wan Y. J.; Ying, Y. L.; Long, Y. T. An Envelope Algorithm for Single Nanoparticles Collision Electrochemistry. Chin. J. Chem. 2021, DOI: https://doi.org/10.1002/cjoc.202100079.
- 45 Gaven, F.; Marin, P.; Claeysen, S. Primary Culture of Mouse Dopaminergic Neurons. J. Visualized Exp. 2014, 91, e51751.
- 46 Mosharov, E. V.; Sulzer, D. Analysis of Exocytotic Events Recorded by Amperometry. Nat. Methods 2005, 2, 651–658.




