Optogenetic Control of Phosphatidylinositol (3,4,5)-Triphosphate Production by Light-Sensitive Cryptochrome Proteins on the Plasma Membrane
Main observation and conclusion
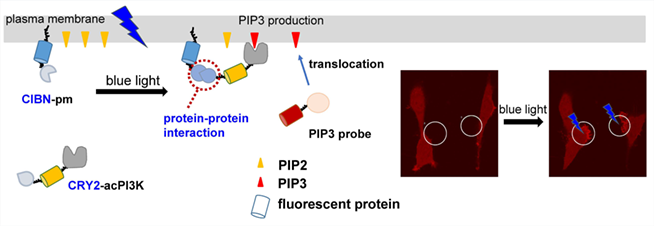
Phosphatidylinositol (3,4,5)-triphosphate (PIP3), acting as a fundamental second messenger, is emerging as a promising biomarker for disease diagnosis and prognosis. However, the real time analysis of phosphoinositide in living cells remains a key challenge owing to the low basal abundance and its fast metabolic rate. Herein, we design an optogenetic system that uses light sensitive protein-protein interaction between Arabidopsis cryptochrome 2 (CRY2) and CIB1 to spatiotemporally visualize the PIP3 production with sub-second timescale. In this system, a CIBN is anchored on the plasma membrane, whereas a CRY2 fused with a constitutively active PI3-kinase (acPI3K) would be driven from the cytosol to the membrane by the blue-light-activated CRY2-CIB1 interaction upon light irradiation. The PIP3 production is visualized via a fused fluorescent protein by the translocation of a Pleckstrin Homology (PH) domain (GRP1) from the cytosol to the plasma membrane with high specificity. We demonstrated the fast dynamics and reversibility of the optogenetic system initiated PIP3 synthesis on the plasma membrane. Notably, the real-time cell movements were also observed upon localized light stimulation. The established optogenetic method provides a novel spatiotemporal strategy for specific PIP3 visualization, which is beneficial to improve the understanding of PIP3 functions.