Synthesis and Antifungal Activity of Norbornene Carboxamide/sulfonamide Derivatives as Potential Fungicides Targeting Laccase
Zi-Hui Yang
Jiangsu Co-Innovation Center of Efficient Processing and Utilization of Forest Resources, Jiangsu Provincial Key Lab for the Chemistry and Utilization of Agro-forest Biomass, Jiangsu Key Lab of Biomass-Based Green Fuels and Chemicals, College of Chemical Engineering, Nanjing Forestry University, Nanjing, 210037 China
Contribution: Writing - original draft (lead), Writing - review & editing (equal)
Search for more papers by this authorYi-Gui Qiu
Jiangsu Co-Innovation Center of Efficient Processing and Utilization of Forest Resources, Jiangsu Provincial Key Lab for the Chemistry and Utilization of Agro-forest Biomass, Jiangsu Key Lab of Biomass-Based Green Fuels and Chemicals, College of Chemical Engineering, Nanjing Forestry University, Nanjing, 210037 China
Contribution: Investigation (equal)
Search for more papers by this authorDao-Jun Jin
Jiangsu Co-Innovation Center of Efficient Processing and Utilization of Forest Resources, Jiangsu Provincial Key Lab for the Chemistry and Utilization of Agro-forest Biomass, Jiangsu Key Lab of Biomass-Based Green Fuels and Chemicals, College of Chemical Engineering, Nanjing Forestry University, Nanjing, 210037 China
Contribution: Investigation (equal)
Search for more papers by this authorYi-Ming Zheng
Jiangsu Co-Innovation Center of Efficient Processing and Utilization of Forest Resources, Jiangsu Provincial Key Lab for the Chemistry and Utilization of Agro-forest Biomass, Jiangsu Key Lab of Biomass-Based Green Fuels and Chemicals, College of Chemical Engineering, Nanjing Forestry University, Nanjing, 210037 China
Contribution: Investigation (equal)
Search for more papers by this authorCorresponding Author
Jia Li
School of Foreign Languages, Nanjing Xiaozhuang University, Nanjing, 211171 China
Contribution: Writing - review & editing (equal)
Search for more papers by this authorCorresponding Author
Wen Gu
Jiangsu Co-Innovation Center of Efficient Processing and Utilization of Forest Resources, Jiangsu Provincial Key Lab for the Chemistry and Utilization of Agro-forest Biomass, Jiangsu Key Lab of Biomass-Based Green Fuels and Chemicals, College of Chemical Engineering, Nanjing Forestry University, Nanjing, 210037 China
Contribution: Writing - original draft (lead), Writing - review & editing (equal)
Search for more papers by this authorZi-Hui Yang
Jiangsu Co-Innovation Center of Efficient Processing and Utilization of Forest Resources, Jiangsu Provincial Key Lab for the Chemistry and Utilization of Agro-forest Biomass, Jiangsu Key Lab of Biomass-Based Green Fuels and Chemicals, College of Chemical Engineering, Nanjing Forestry University, Nanjing, 210037 China
Contribution: Writing - original draft (lead), Writing - review & editing (equal)
Search for more papers by this authorYi-Gui Qiu
Jiangsu Co-Innovation Center of Efficient Processing and Utilization of Forest Resources, Jiangsu Provincial Key Lab for the Chemistry and Utilization of Agro-forest Biomass, Jiangsu Key Lab of Biomass-Based Green Fuels and Chemicals, College of Chemical Engineering, Nanjing Forestry University, Nanjing, 210037 China
Contribution: Investigation (equal)
Search for more papers by this authorDao-Jun Jin
Jiangsu Co-Innovation Center of Efficient Processing and Utilization of Forest Resources, Jiangsu Provincial Key Lab for the Chemistry and Utilization of Agro-forest Biomass, Jiangsu Key Lab of Biomass-Based Green Fuels and Chemicals, College of Chemical Engineering, Nanjing Forestry University, Nanjing, 210037 China
Contribution: Investigation (equal)
Search for more papers by this authorYi-Ming Zheng
Jiangsu Co-Innovation Center of Efficient Processing and Utilization of Forest Resources, Jiangsu Provincial Key Lab for the Chemistry and Utilization of Agro-forest Biomass, Jiangsu Key Lab of Biomass-Based Green Fuels and Chemicals, College of Chemical Engineering, Nanjing Forestry University, Nanjing, 210037 China
Contribution: Investigation (equal)
Search for more papers by this authorCorresponding Author
Jia Li
School of Foreign Languages, Nanjing Xiaozhuang University, Nanjing, 211171 China
Contribution: Writing - review & editing (equal)
Search for more papers by this authorCorresponding Author
Wen Gu
Jiangsu Co-Innovation Center of Efficient Processing and Utilization of Forest Resources, Jiangsu Provincial Key Lab for the Chemistry and Utilization of Agro-forest Biomass, Jiangsu Key Lab of Biomass-Based Green Fuels and Chemicals, College of Chemical Engineering, Nanjing Forestry University, Nanjing, 210037 China
Contribution: Writing - original draft (lead), Writing - review & editing (equal)
Search for more papers by this authorAbstract
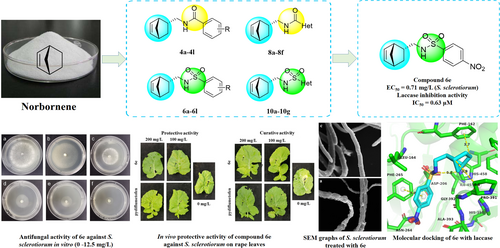
To explore more potential fungicides with new scaffolds, thirty-seven norbornene carboxamide/sulfonamide derivatives were designed, synthesized, and assayed for inhibitory activity against six plant pathogenic fungi and oomycetes. The preliminary antifungal assay suggested that the title derivatives showed moderate to good antifungal activity against six plant pathogens. Especially, compound 6 e presented excellent in vitro antifungal activity against Sclerotinia sclerotiorum (EC50=0.71 mg/L), which was substantially stronger than pydiflumetofen. In vivo antifungal assay indicated 6 e displayed prominent protective and curative effects on rape leaves infected by S. sclerotiorum. The preliminary mechanism research displayed that 6 e could damage the surface morphology and inhibit the sclerotia formation of S. sclerotiorum. In addition, the in vitro enzyme inhibition bioassay indicated that 6 e displayed pronounced laccase inhibition activity (IC50=0.63 μM), much stronger than positive control cysteine. Molecular docking elucidated the binding modes between 6 e and laccase. The bioassay results and mechanism investigation demonstrated that this class of norbornene carboxamide/sulfonamide derivatives could be promising laccase inhibitors for novel fungicide development.
Graphical Abstract
Conflict of interests
The authors declare no conflict of interest.
Supporting Information
As a service to our authors and readers, this journal provides supporting information supplied by the authors. Such materials are peer reviewed and may be re-organized for online delivery, but are not copy-edited or typeset. Technical support issues arising from supporting information (other than missing files) should be addressed to the authors.
| Filename | Description |
|---|---|
| cbdv202302033-sup-0001-misc_information.pdf5.5 MB | Supporting Information |
Please note: The publisher is not responsible for the content or functionality of any supporting information supplied by the authors. Any queries (other than missing content) should be directed to the corresponding author for the article.
References
- 1J. W. Peng, X. D. Yin, H. Li, K. Y. Ma, Z. J. Zhang, R. Zhou, Y. L. Wang, G. F. Hu, Y. Q. Liu, J. Agric. Food Chem. 2021, 69, 4604–4614.
- 2Q. Cheng, W. Jia, C. Hu, G. Shi, D. Yang, M. Cai, T. Zhan, Y. Tang, Y. Zhou, X. Sun, X. Zhao, Environ. Pollut. 2020, 265, 114827.
- 3Y. Duan, C. Ge, S. Liu, C. Chen, M. Zhou, Pestic. Biochem. Physiol. 2013, 106, 61–67.
- 4Z. Wang, Q. Peng, Y. Hou, X. Gao, S. Zhong, Y. Fang, Pest Manage. Sci. 2020, 76, 2525–2536.
- 5H. Xu, L. Fan, Eur. J. Med. Chem. 2011, 46, 364–369.
- 6Q. Wang, Y. S. Mao, S. X. Li, T. Li, J. X. Wang, M. G. Zhou, Y. B. Duan, J. Agric. Food Chem. 2022, 70, 7039–7048.
- 7A. M. Mayer, R. C. Staples, Phytochemistry. 2002, 60, 551–565.
- 8H. Yoshida, J. Chem. Soc. Trans. 1883, 43, 472–486.
- 9S. R. Couto, J. L. Toca Herrera, Biotechnol. Adv. 2006, 24, 500–513.
- 10M. Smith, C. F. Thurston, D. A. Wood, Multi-copper Oxidases 1997, 201–224.
- 11M. C. N. Saparrat, F. Guillén, A. M. Arambarri, A. T. Martínez, M. J. Martínez, Appl. Environ. Microbiol. 2002, 68, 1534–1540.
- 12A. A. Bell, M. H. Wheeler, Annu. Rev. Phytopathol. 1986, 24, 411–451.
- 13Y. Sakamoto, K. Nakade, S. Sato, A. Yoshimi, K. Sasaki, N. Konno, Fungal Biol. Rev. 2018, 122, 1192–1200.
- 14Z. W. Wang, Q. Peng, X. Gao, S. Zhong, Y. Fang, X. L. Yang, Y. Ling, X. L. Liu, J. Agric. Food Chem. 2020, 68, 5318–5326.
- 15X. M. Zhang, H. Xu, H. F. Su, X. L. Yang, T. D. Sun, X. X. Lu, F. S. Shi, H. X. Duan, X. L. Liu, Y. Ling, J. Agric. Food Chem. 2022, 70, 1776–1787.
- 16T. D. Sun, X. Y. Jin, X. M. Zhang, X. M. Lu, C. K. Wang, J. L. Cui, H. Xu, X. L. Yang, X. L. Liu, L. Zhang, Y. Ling, J. Agric. Food Chem. 2022, 70, 14367–14376.
- 17H. Xu, X. X. Lu, T. D. Sun, Q. He, Y. Qi, Y. F. Lin, X. L. Yang, L. Zhang, Y. Ling, X. M. Zhang, J. Mol. Struct. 2023, 1285, 135526.
- 18T. D. Sun, X. Y. Jin, X. M. Zhang, X. X. Lu, H. Xu, J. L. Cui, X. L. Yang, X. L. Liu, L. Zhang, Y. Ling, Pest Manage. Sci. 2023, 79, 3773–3784.
- 19X. B. Sun, Z. H. Yang, D. J. Jin, Y. G. Qiu, W. Gu, Pest Manage. Sci. 2023, 79, 2469–2481.
- 20M. Chen, W. P. Zou, Z. Cai, C. L. Chen, Polym. Chem. 2015, 6, 2669–2676.
- 21L. X. Pei, Y. Tang, H. Y. Gao, Polymer 2016, 8, 69.
- 22J. F. Meng, X. Li, X. F. Ni, Z. Q. Shen, RSC Adv. 2016, 6, 19351–19356.
- 23S. T. Nguyen, L. K. Johnson, R. H. Grubbs, J. W. Ziller, J. Am. Chem. Soc. 1992, 114, 3974–3975.
- 24K. Cao, B. Yuan, X. Liu, M. F. Wu, Z. Yao, Prog. Chem. 2017, 29, 605–616.
- 25G. Calvo-Martin, D. Plano, N. Martinez-Saez, C. Aydillo, E. Moreno, S. Espuelas, C. Sanmartin, Pharmaceuticals 2022, 15, 1465.
- 26B. Luo, Y. L. Ning, J. Agric. Food Chem. 2022, 70, 957–975.
- 27M. I. Shatirovaa, M. M. Movsumzadeb, U. S. Dzhafarovab, Y. G. Avdeev, Pet. Chem. 2019, 59, 220–227.
- 28E. H. Mammadbayli, G. E. Hajiyeva, S. I. Ibrahimli, N. A. Cafarova, Russ. J. Appl. Chem. 2019, 92, 1161–1169.
- 29X. Wang, A. Wang, L. Qiu, M. Chen, A. Lu, G. Li, J. Agric. Food Chem. 2020, 68, 14426–14437.
- 30Y. Y. Wu, W. B. Shao, J. J. Zhu, Z. Q. Long, L. W. Liu, P. Y. Wang, J. Agric. Food Chem. 2019, 67, 13892–13903.
- 31Y. P. Hou, X. W. Mao, J. X. Wang, S. W. Zhan, M. G. Zhou, Crop Prot. 2017, 96, 237–244.
- 32J. Lin, S. Zhou, J. X. Xu, W. Q. Yao, G. F. Hao, Y. T. Li, J. Agric. Food Chem. 2020, 68, 6792–6801.
- 33W. J. Zhao, S. J. Zheng, J. W. Zou, Y. S. Liang, C. Zhao, H. H. Xu, J. Agric. Food Chem. 2021, 69, 5798–5803.
- 34B. Andrew, R. Ramakrishna, F. Dietrichm, W. N. Peter, US2008/0027246A1, 2008.
- 35G. M. Sheldrick, SXELXS-97 and SXELXL-97, Program for X-ray crystal structure solution; University of Göttingen, Göttingen (Germany), 1997.