Enantioselective Divergent Cyclization of Amide-Type 1,6-Enynes Enabled by H–[Pd]–CN Catalysis
Graphical Abstract
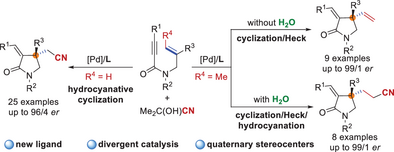
Three distinctive asymmetric 1,6-enyne cyclization strategies, namely, hydrocyanative cyclization, cyclization/Heck reaction, and cyclization/Heck/hydrocyanation, have been developed, providing a modular synthetic platform for various chiral γ-lactams bearing a quaternary stereogenic center. Furthermore, preliminary mechanistic studies, including DFT calculations, are also conducted.
Abstract
Enantioselective cyclization of 1,6-enynes has been extensively developed as it offers an effective way to obtain chiral cyclic compounds. Herein, we report a divergent palladium-catalyzed enantioselective cyclization of N-allyl-substituted propiolamides, which allows for the efficient synthesis of chiral γ-lactams bearing a quaternary stereogenic center. Three distinctive cyclization strategies, namely, hydrocyanative cyclization, cyclization/Heck reaction, and cyclization/Heck/hydrocyanation, were successfully developed. The product can be selected by using different initial substrates under the same catalytic conditions or by fine-tuning the reaction conditions for the same initial substrate. This catalytic system boasts broad substrate scope and wide functional-group compatibility, where a diverse range of substituents on the aryl group and amide nitrogen atom were tolerated. The combination of experimental studies and theoretical calculations revealed the fundamental role of the ligand in regioselectivity and the role of H2O in the sequential cyclization/Heck/hydrocyanation reactions. H2O can lower the energy barrier of the reductive elimination step, thus promoting the reaction toward the target product formation.
Conflict of Interests
The authors declare no conflict of interest.
Open Research
Data Availability Statement
The data that support the findings of this study are available in the Supporting Information of this article.